Assessment of Dronabinol and Its Stereo-Isomers1,2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And Anxiety-Related Behavioral Effects of Repeated Nicotine As a Stressor: the Role of Cannabinoid Receptors Tamaki Hayase
Hayase BMC Neuroscience 2013, 14:20 http://www.biomedcentral.com/1471-2202/14/20 RESEARCH ARTICLE Open Access Working memory- and anxiety-related behavioral effects of repeated nicotine as a stressor: the role of cannabinoid receptors Tamaki Hayase Abstract Background: Like emotional symptoms such as anxiety, modulations in working memory are among the frequently-reported but controversial psychiatric symptoms associated with nicotine (NC) administration. In the present study, repeated NC-induced modulations in working memory, along with concurrently-observed anxiety- related behavioral alterations, were investigated in mice, and compared with the effects of a typical cognition- impairing stressor, immobilization stress (IM). Furthermore, considering the structural and functional contributions of brain cannabinoid (CB) receptors in NC-induced psychiatric symptoms including emotional symptoms, the interactive effects of brain CB receptor ligands (CB ligands) and NC and/or IM on the working memory- and anxiety-related behaviors were examined. Results: Statistically significant working memory impairment-like behavioral alterations in the Y-maze test and anxiety-like behavioral alterations in the elevated plus-maze (EPM) test were observed in the groups of mice treated with 0.8 mg/kg NC (subcutaneous (s.c.) 0.8 mg/kg treatment, 4 days) and/or IM (10 min treatment, 4 days). In the group of mice treated with NC plus IM (NC-IM group), an enhancement of the behavioral alterations was observed. Among the CB type 1 (CB1) antagonist AM 251 (AM), the non-selective CB agonist CP 55,940 (CP), and the CB1 partial agonist/antagonist virodhamine (VD), significant recovering effects were provided by AM (0.2-2.5 mg/kg) and VD (5 mg/kg) against the working memory impairment-like behaviors, whereas significant anxiolytic-like effects (recoveries from both attenuated percentage of entries into open arms and attenuated percentage of time spent on open arms) were provided by VD (1–10 mg/kg) and CP (2 mg/kg) against the anxiety-like behaviors. -

Genl:VE 1970 © World Health Organization 1970
Nathan B. Eddy, Hans Friebel, Klaus-Jiirgen Hahn & Hans Halbach WORLD HEALTH ORGANIZATION ORGANISATION .MONDIALE DE LA SANT~ GENl:VE 1970 © World Health Organization 1970 Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. Nevertheless governmental agencies or learned and professional societies may reproduce data or excerpts or illustrations from them without requesting an authorization from the World Health Organization. For rights of reproduction or translation of WHO publications in toto, application should be made to the Division of Editorial and Reference Services, World Health Organization, Geneva, Switzerland. The World Health Organization welcomes such applications. Authors alone are responsible for views expressed in signed articles. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Director-General of the World Health Organization concerning the legal status of any country or territory or of its authorities, or concerning the delimitation of its frontiers. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. © Organisation mondiale de la Sante 1970 Les publications de l'Organisation mondiale de la Sante beneficient de la protection prevue par les dispositions du Protocole n° 2 de la Convention universelle pour la Protection du Droit d'Auteur. Les institutions gouvernementales et les societes savantes ou professionnelles peuvent, toutefois, reproduire des donnees, des extraits ou des illustrations provenant de ces publications, sans en demander l'autorisation a l'Organisation mondiale de la Sante. Pour toute reproduction ou traduction integrate, une autorisation doit etre demandee a la Division des Services d'Edition et de Documentation, Organisation mondiale de la Sante, Geneve, Suisse. -

Potential Cannabis Antagonists for Marijuana Intoxication
Central Journal of Pharmacology & Clinical Toxicology Bringing Excellence in Open Access Review Article *Corresponding author Matthew Kagan, M.D., Cedars-Sinai Medical Center, 8730 Alden Drive, Los Angeles, CA 90048, USA, Tel: 310- Potential Cannabis Antagonists 423-3465; Fax: 310.423.8397; Email: Matthew.Kagan@ cshs.org Submitted: 11 October 2018 for Marijuana Intoxication Accepted: 23 October 2018 William W. Ishak, Jonathan Dang, Steven Clevenger, Shaina Published: 25 October 2018 Ganjian, Samantha Cohen, and Matthew Kagan* ISSN: 2333-7079 Cedars-Sinai Medical Center, USA Copyright © 2018 Kagan et al. Abstract OPEN ACCESS Keywords Cannabis use is on the rise leading to the need to address the medical, psychosocial, • Cannabis and economic effects of cannabis intoxication. While effective agents have not yet been • Cannabinoids implemented for the treatment of acute marijuana intoxication, a number of compounds • Antagonist continue to hold promise for treatment of cannabinoid intoxication. Potential therapeutic • Marijuana agents are reviewed with advantages and side effects. Three agents appear to merit • Intoxication further inquiry; most notably Cannabidiol with some evidence of antipsychotic activity • THC and in addition Virodhamine and Tetrahydrocannabivarin with a similar mixed receptor profile. Given the results of this research, continued development of agents acting on cannabinoid receptors with and without peripheral selectivity may lead to an effective treatment for acute cannabinoid intoxication. Much work still remains to develop strategies that will interrupt and reverse the effects of acute marijuana intoxication. ABBREVIATIONS Therapeutic uses of cannabis include chronic pain, loss of appetite, spasticity, and chemotherapy-associated nausea and CBD: Cannabidiol; CBG: Cannabigerol; THCV: vomiting [8]. Recreational cannabis use is on the rise with more Tetrahydrocannabivarin; THC: Tetrahydrocannabinol states approving its use and it is viewed as no different from INTRODUCTION recreational use of alcohol or tobacco [9]. -

Cannabinoids and Reproduction: a Lasting and Intriguing History
Pharmaceuticals 2010, 3, 3275-3323; doi:10.3390/ph3103275 OPEN ACCESS pharmaceuticals ISSN 1424-8247 www.mdpi.com/journal/pharmaceuticals Review Cannabinoids and Reproduction: A Lasting and Intriguing History Giovanna Cacciola 1,†, Rosanna Chianese 1,†, Teresa Chioccarelli 1,†, Vincenza Ciaramella 1, Silvia Fasano 1, Riccardo Pierantoni 1,*, Rosaria Meccariello 2,# and Gilda Cobellis 1,# 1 Dipartimento di Medicina Sperimentale, Sez. “F. Bottazzi”, Seconda Università degli Studi di Napoli, Napoli, Italy 2 Dipartimento di Studi delle Istituzioni e dei Sistemi Territoriali, Università di Napoli “Parthenope”, Napoli, Italy † These authors contributed equally to the paper. # Equally senior authors. * Author to whom correspondence should be addressed; E-Mail: [email protected]. Received: 12 August 2010; in revised form: 9 September 2010 / Accepted: 21 October 2010 / Published: 25 October 2010 Abstract: Starting from an historical overview of lasting Cannabis use over the centuries, we will focus on a description of the cannabinergic system, with a comprehensive analysis of chemical and pharmacological properties of endogenous and synthetic cannabimimetic analogues. The metabolic pathways and the signal transduction mechanisms, activated by cannabinoid receptors stimulation, will also be discussed. In particular, we will point out the action of cannabinoids and endocannabinoids on the different neuronal networks involved in reproductive axis, and locally, on male and female reproductive tracts, by emphasizing the pivotal role played -

Tasty THC: Promises and Challenges of Cannabis Edibles
RTI Press Occasional Paper November 2016 Tasty THC: Promises and Challenges of Cannabis Edibles Daniel G. Barrus, Kristen L. Capogrossi, Sheryl C. Cates, Camille K. Gourdet, Nicholas C. Peiper, Scott P. Novak, Timothy W. Lefever, and Jenny L. Wiley RTI Press publication OP-0035-1611 This PDF document was made available from www.rti.org as a public service of RTI International. More information about RTI Press can be found at http://www.rti.org/rtipress. RTI International is an independent, nonprofit research organization dedicated to improving the human condition by turning knowledge into practice. The RTI Press mission is to disseminate information about RTI research, analytic tools, and technical expertise to a national and international audience. RTI Press publications are peer- reviewed by at least two independent substantive experts and one or more Press editors. Suggested Citation Barrus, D.G., Capogrossi, K.L., Cates, S.C., Gourdet, C.K., Peiper, N.C., Novak, S.P., Lefever, T.W., and Wiley, J.L. (2016). Tasty THC: Promises and Challenges of Cannabis Edibles. RTI Press Publication No. OP-0035-1611. Research Triangle Park, NC: RTI Press. http://dx.doi.org /10.3768/rtipress.2016.op.0035.1611 This publication is part of the RTI Press Research Report series. Occasional Papers are scholarly essays on policy, methods, or other topics relevant to RTI areas of research or technical focus. RTI International 3040 East Cornwallis Road PO Box 12194 ©2016 RTI International. All rights reserved. Credit must be provided to the author and source of the Research Triangle Park, NC publication when the content is quoted. -

DRONABINOL What Should You Do If You FORGET a What Other PRECAUTIONS Should Dose? You Follow While Using This Drug?
DRONABINOL What should you do if you FORGET a What other PRECAUTIONS should dose? you follow while using this drug? Other NAMES : Marinol , delta – 9 - If you miss a dose of dronabinol, take it Before starting dronabinol, inform your tetrahydrocannabinol as soon as possible. However, if it is doctor if you have already had in the time for your next dose, do not double past adverse reactions to dronabinol, WHY is this drug prescribed? the dose, just carry on with your regular nabilone (Cesamet ) or marijuana schedule. (pot). Also, notify your doctor if you are Dronabinol is a narcotic drug used to allergic to sesame oil, if you have heart treat severe nausea and vomiting. It is What ADVERSE EFFECTS can this disease (hypertension, abnormal in a family of drugs called cannabinoids drug cause? What should you do heartbeat, angina), or a history of (eg. marijuana). It is also used to about them? emotional disorders such as depression, increase appetite and weight gain. bipolar disease (manic depression), and Dronabinol can cause unsteadiness, schizophrenia (psychosis, HOW should this drug be taken? dizziness, difficulty concentrating, hallucinations). drowsiness, euphoria, abnormal Dronabinol is available in 2.5 mg, 5 mg thinking, fatigue, anxiety, confusion, As this drug may cause some people to and 10 mg capsules. To increase hallucinations, and paranoia. become dizzy, drowsy or less alert than appetite and body weight, the usual Nausea, vomiting, abdominal pain, and normal, you should NOT drive, use initial dose is 2.5 mg twice daily. facial redness may also occur. machinery or do anything else that Depending on the results obtained, your could be dangerous if you are dizzy or doctor might decide to slowly increase These effects may go away during are not alert. -

Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back
International Journal of Molecular Sciences Review Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back Osnat Almogi-Hazan * and Reuven Or Laboratory of Immunotherapy and Bone Marrow Transplantation, Hadassah Medical Center, The Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem 91120, Israel; [email protected] * Correspondence: [email protected] Received: 21 May 2020; Accepted: 19 June 2020; Published: 23 June 2020 Abstract: The Cannabis plant contains numerous components, including cannabinoids and other active molecules. The phyto-cannabinoid activity is mediated by the endocannabinoid system. Cannabinoids affect the nervous system and play significant roles in the regulation of the immune system. While Cannabis is not yet registered as a drug, the potential of cannabinoid-based medicines for the treatment of various conditions has led many countries to authorize their clinical use. However, the data from basic and medical research dedicated to medical Cannabis is currently limited. A variety of pathological conditions involve dysregulation of the immune system. For example, in cancer, immune surveillance and cancer immuno-editing result in immune tolerance. On the other hand, in autoimmune diseases increased immune activity causes tissue damage. Immuno-modulating therapies can regulate the immune system and therefore the immune-regulatory properties of cannabinoids, suggest their use in the therapy of immune related disorders. In this contemporary review, we discuss the roles of the endocannabinoid system in immunity and explore the emerging data about the effects of cannabinoids on the immune response in different pathologies. In addition, we discuss the complexities of using cannabinoid-based treatments in each of these conditions. -

DRONABINOL- Dronabinol Capsule Actavis Pharma, Inc
DRONABINOL- dronabinol capsule Actavis Pharma, Inc. ---------- DRONABINOL Capsules, USP 2.5 mg, 5 mg, 10 mg CIII Rx only DESCRIPTION Dronabinol is a cannabinoid designated chemically as (6aR-trans)-6a,7,8,10a-tetrahydro-6,6,9- trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol. Dronabinol has the following empirical and structural formulas: Dronabinol, the active ingredient in dronabinol capsules, USP, is synthetic delta-9-tetrahydrocannabinol (delta-9-THC). Delta-9-tetrahydrocannabinol is also a naturally occurring component of Cannabis sativa L. (Marijuana). Dronabinol is a light yellow resinous oil that is sticky at room temperature and hardens upon refrigeration. Dronabinol is insoluble in water and is formulated in sesame oil. It has a pKa of 10.6 and an octanol-water partition coefficient: 6,000:1 at pH 7. Capsules for oral administration: Dronabinol capsules are supplied as round, soft gelatin capsules containing either 2.5 mg, 5 mg, or 10 mg dronabinol. Each dronabinol capsule strength is formulated with the following inactive ingredients: 2.5 mg capsule contains gelatin, glycerin, sesame oil, and titanium dioxide; 5 mg capsule contains iron oxide red and iron oxide black, gelatin, glycerin, sesame oil, and titanium dioxide; 10 mg capsule contains iron oxide red and iron oxide yellow, gelatin, glycerin, sesame oil, and titanium dioxide. CLINICAL PHARMACOLOGY Dronabinol is an orally active cannabinoid which, like other cannabinoids, has complex effects on the central nervous system (CNS), including central sympathomimetic activity. Cannabinoid receptors have been discovered in neural tissues. These receptors may play a role in mediating the effects of dronabinol and other cannabinoids. -

The Rise and Decline of Cannabis Prohibition the History of Cannabis in the UN Drug Control System and Options for Reform
TRANSNATIONAL I N S T I T U T E THE RISE AND DECLINE OF CANNABIS PROHIBITION THE HISTORY OF CANNABIS IN THE UN DruG CONTROL SYSTEM AND OPTIONS FOR REFORM 3 The Rise and Decline of Cannabis Prohibition Authors Dave Bewley-Taylor Tom Blickman Martin Jelsma Copy editor David Aronson Design Guido Jelsma www.guidojelsma.nl Photo credits Hash Marihuana & Hemp Museum, Amsterdam/ Barcelona Floris Leeuwenberg Pien Metaal UNOG Library/League of Nations Archives UN Photo Printing Jubels, Amsterdam Contact Transnational Institute (TNI) De Wittenstraat 25 1052 AK Amsterdam Netherlands Tel: +31-(0)20-6626608 Fax: +31-(0)20-6757176 [email protected] www.tni.org/drugs www.undrugcontrol.info www.druglawreform.info Global Drug Policy Observatory (GDPO) Research Institute for Arts and Humanities Rooms 201-202 James Callaghan Building Swansea University Financial contributions Singleton Park, Swansea SA2 8PP Tel: +44-(0)1792-604293 This report has been produced with the financial www.swansea.ac.uk/gdpo assistance of the Hash Marihuana & Hemp Museum, twitter: @gdpo_swan Amsterdam/Barcelona, the Open Society Foundations and the Drug Prevention and Information Programme This is an Open Access publication distributed under (DPIP) of the European Union. the terms of the Creative Commons Attribution License The contents of this publication are the sole responsibility (http://creativecommons.org/licenses/by/2.0), which of TNI and GDPO and can under no circumstances be permits unrestricted use, distribution, and reproduction regarded as reflecting the position of the donors. in any medium, provided the original work is properly cited. TNI would appreciate receiving a copy of the text in which this document is used or cited. -

The Use of Cannabinoids in Animals and Therapeutic Implications for Veterinary Medicine: a Review
Veterinarni Medicina, 61, 2016 (3): 111–122 Review Article doi: 10.17221/8762-VETMED The use of cannabinoids in animals and therapeutic implications for veterinary medicine: a review L. Landa1, A. Sulcova2, P. Gbelec3 1Faculty of Medicine, Masaryk University, Brno, Czech Republic 2Central European Institute of Technology, Masaryk University, Brno, Czech Republic 3Veterinary Hospital and Ambulance AA Vet, Prague, Czech Republic ABSTRACT: Cannabinoids/medical marijuana and their possible therapeutic use have received increased atten- tion in human medicine during the last years. This increased attention is also an issue for veterinarians because particularly companion animal owners now show an increased interest in the use of these compounds in veteri- nary medicine. This review sets out to comprehensively summarise well known facts concerning properties of cannabinoids, their mechanisms of action, role of cannabinoid receptors and their classification. It outlines the main pharmacological effects of cannabinoids in laboratory rodents and it also discusses examples of possible beneficial use in other animal species (ferrets, cats, dogs, monkeys) that have been reported in the scientific lit- erature. Finally, the article deals with the prospective use of cannabinoids in veterinary medicine. We have not intended to review the topic of cannabinoids in an exhaustive manner; rather, our aim was to provide both the scientific community and clinical veterinarians with a brief, concise and understandable overview of the use of cannabinoids in veterinary -

Sympathoadrenergic Modulation of Hematopoiesis: a Review of Available Evidence and of Therapeutic Perspectives
REVIEW published: 05 August 2015 doi: 10.3389/fncel.2015.00302 Sympathoadrenergic modulation of hematopoiesis: a review of available evidence and of therapeutic perspectives Marco Cosentino*, Franca Marino and Georges J. M. Maestroni Center for Research in Medical Pharmacology, University of Insubria, Varese, Italy Innervation of the bone marrow (BM) has been described more than one century ago, however the first in vivo evidence that sympathoadrenergic fibers have a role in hematopoiesis dates back to less than 25 years ago. Evidence has since increased showing that adrenergic nerves in the BM release noradrenaline and possibly also dopamine, which act on adrenoceptors and dopaminergic receptors (DR) expressed on hematopoietic cells and affect cell survival, proliferation, migration and engraftment ability. Remarkably, dysregulation of adrenergic fibers to the BM is associated with hematopoietic disturbances and myeloproliferative disease. Several adrenergic and dopaminergic agents are already in clinical use for non-hematological indications and with a usually favorable risk-benefit profile, and are therefore potential candidates for Edited by: non-conventional modulation of hematopoiesis. Wanda Lattanzi, Università Cattolica del Sacro Cuore, Keywords: dopamine, noradrenaline, adrenaline, adrenoceptors, dopaminergic receptors, hematopoiesis, Italy neuroimmune phamacology, drug repurposing Reviewed by: Sujit Basu, Introduction Ohio State University, USA Tsvee Lapidot, Weizmann Institute of Science, Israel The term ‘‘niche’’, derived from the Latin word ‘‘mytilus’’ (mussel), has eventually come to designate a shallow recess in a wall, as for a statue or other decorative object, in view of the *Correspondence: similarity with the shape of a seashell, and broadly a place suitable or appropriate for a person or Marco Cosentino, Center for Research in Medical thing. -
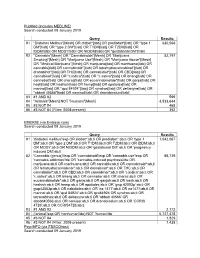
Diabetes Mellitus
PubMed (includes MEDLINE) Search conducted 09 January 2019 Query Results #1 "Diabetes Mellitus"[Mesh] OR diabet*[tiab] OR prediabet*[tiab] OR "type 1 630,566 DM"[tiab] OR "type 2 DM"[tiab] OR T1DM[tiab] OR T2DM[tiab] OR IDDM[tiab] OR MODY[tiab] OR NIDDM[tiab] OR "gestational DM"[tiab] #2 "Cannabis"[Mesh] OR "Cannabinoids"[Mesh] OR "Marijuana 52,197 Smoking"[Mesh] OR "Marijuana Use"[Mesh] OR "Marijuana Abuse"[Mesh] OR "Medical Marijuana"[Mesh] OR marijuana[tiab] OR marihuana[tiab] OR cannabis[tiab] OR cannabinoid*[tiab] OR tetrahydrocannabinol*[tiab] OR dronabinol*[tiab] OR THC[tiab] OR cannabidiol*[tiab] OR CBD[tiab] OR cannabinol*[tiab] OR "c.indica"[tiab] OR "c.sativa"[tiab] OR bhang[tiab] OR cannador[tiab] OR charas[tiab] OR eucannabinolide*[tiab] OR ganja[tiab] OR hash[tiab] OR hashish[tiab] OR hemp[tiab] OR epidiolex[tiab] OR marinol[tiab] OR "qcd 84924"[tiab] OR syndros[tiab] OR deltanyne[tiab] OR "abbott 40566"[tiab] OR namisol[tiab] OR dronabinolum[tiab] #3 #1 AND #2 599 #4 "Animals"[Mesh] NOT "Humans"[Mesh] 4,533,644 #5 #3 NOT #4 468 #6 #3 NOT #4 (Filter: 2008-present) 352 EMBASE (via Embase.com) Search conducted 09 January 2019 Query Results #1 'diabetes mellitus'/exp OR diabet*:ab,ti OR prediabet*:ab,ti OR 'type 1 1,042,067 DM':ab,ti OR 'type 2 DM':ab,ti OR T1DM:ab,ti OR T2DM:ab,ti OR IDDM:ab,ti OR MODY:ab,ti OR NIDDM:ab,ti OR 'gestational DM':ab,ti OR 'pregnancy induced DM':ab,ti #2 'Cannabis (genus)'/exp OR 'cannabinoid'/exp OR 'cannabis use'/exp OR 88,739 'cannabis addiction'/de OR 'cannabis-induced psychosis'/de OR marijuana:ab,ti