Cannabinoids for Medical Use: a Systematic Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Domains: 1. Optimizing Medication Use, Using Front- and Back Office Strategies 2
A SUPPLEMENTARY TABLES Table S1: Domains and themes in the action plan of the Belgian Government (2015-2020) Domains: 1. Optimizing medication use, using front- and back office strategies 2. Continuity of pharmacotherapy in light of transmural care 3. Scientific skills of the hospital pharmacist 4. Transfer of information to and communication with the patient Themes: 2015: anchoring the minimal conditions for the application of clinical pharmacy 2016: development of a structured method for the anamnesis, registration and communication of the medication on admission and discharge 2017: application of clinical pharmacy for specific therapies 2018: application of risk screening for patient groups 2019: application of risk screening for medication groups or pathologies 2020: evaluation of the development of clinical pharmacy in the Belgian hospitals and evaluation of the action plan 2015-2020 Table S2: Anatomical and therapeutic classes of the drugs involved in the discrepancies detected after medication reconciliation n (%) Example(s) of discrepancy Gastro-intestinal system 43 (35.2%) antacids 6 O: antacid PRN antihistaminics 1 O: ranitidine 150 mg 1 pd proton pump inhibitors 5 O: pantoprazole 20 mg 1 pd propulsives 3 O: domperidone PRN; N: alizapride laxatives 9 O: macrogol PRN or 1 pd, bisacodyl PRN or 1 pd antipropulsives 2 O: loperamide 1 pd probiotics 1 O: frequent need for probiotics antidiabetics 1 D: dose repaglinide unknown multivitamins 5 O: multivitamins 1 pd vitamin D 5 O: vitamin D 1 per week vitamin B 2 N: vitamin B complex -

Tasty THC: Promises and Challenges of Cannabis Edibles
RTI Press Occasional Paper November 2016 Tasty THC: Promises and Challenges of Cannabis Edibles Daniel G. Barrus, Kristen L. Capogrossi, Sheryl C. Cates, Camille K. Gourdet, Nicholas C. Peiper, Scott P. Novak, Timothy W. Lefever, and Jenny L. Wiley RTI Press publication OP-0035-1611 This PDF document was made available from www.rti.org as a public service of RTI International. More information about RTI Press can be found at http://www.rti.org/rtipress. RTI International is an independent, nonprofit research organization dedicated to improving the human condition by turning knowledge into practice. The RTI Press mission is to disseminate information about RTI research, analytic tools, and technical expertise to a national and international audience. RTI Press publications are peer- reviewed by at least two independent substantive experts and one or more Press editors. Suggested Citation Barrus, D.G., Capogrossi, K.L., Cates, S.C., Gourdet, C.K., Peiper, N.C., Novak, S.P., Lefever, T.W., and Wiley, J.L. (2016). Tasty THC: Promises and Challenges of Cannabis Edibles. RTI Press Publication No. OP-0035-1611. Research Triangle Park, NC: RTI Press. http://dx.doi.org /10.3768/rtipress.2016.op.0035.1611 This publication is part of the RTI Press Research Report series. Occasional Papers are scholarly essays on policy, methods, or other topics relevant to RTI areas of research or technical focus. RTI International 3040 East Cornwallis Road PO Box 12194 ©2016 RTI International. All rights reserved. Credit must be provided to the author and source of the Research Triangle Park, NC publication when the content is quoted. -

DRONABINOL What Should You Do If You FORGET a What Other PRECAUTIONS Should Dose? You Follow While Using This Drug?
DRONABINOL What should you do if you FORGET a What other PRECAUTIONS should dose? you follow while using this drug? Other NAMES : Marinol , delta – 9 - If you miss a dose of dronabinol, take it Before starting dronabinol, inform your tetrahydrocannabinol as soon as possible. However, if it is doctor if you have already had in the time for your next dose, do not double past adverse reactions to dronabinol, WHY is this drug prescribed? the dose, just carry on with your regular nabilone (Cesamet ) or marijuana schedule. (pot). Also, notify your doctor if you are Dronabinol is a narcotic drug used to allergic to sesame oil, if you have heart treat severe nausea and vomiting. It is What ADVERSE EFFECTS can this disease (hypertension, abnormal in a family of drugs called cannabinoids drug cause? What should you do heartbeat, angina), or a history of (eg. marijuana). It is also used to about them? emotional disorders such as depression, increase appetite and weight gain. bipolar disease (manic depression), and Dronabinol can cause unsteadiness, schizophrenia (psychosis, HOW should this drug be taken? dizziness, difficulty concentrating, hallucinations). drowsiness, euphoria, abnormal Dronabinol is available in 2.5 mg, 5 mg thinking, fatigue, anxiety, confusion, As this drug may cause some people to and 10 mg capsules. To increase hallucinations, and paranoia. become dizzy, drowsy or less alert than appetite and body weight, the usual Nausea, vomiting, abdominal pain, and normal, you should NOT drive, use initial dose is 2.5 mg twice daily. facial redness may also occur. machinery or do anything else that Depending on the results obtained, your could be dangerous if you are dizzy or doctor might decide to slowly increase These effects may go away during are not alert. -

DRONABINOL- Dronabinol Capsule Actavis Pharma, Inc
DRONABINOL- dronabinol capsule Actavis Pharma, Inc. ---------- DRONABINOL Capsules, USP 2.5 mg, 5 mg, 10 mg CIII Rx only DESCRIPTION Dronabinol is a cannabinoid designated chemically as (6aR-trans)-6a,7,8,10a-tetrahydro-6,6,9- trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol. Dronabinol has the following empirical and structural formulas: Dronabinol, the active ingredient in dronabinol capsules, USP, is synthetic delta-9-tetrahydrocannabinol (delta-9-THC). Delta-9-tetrahydrocannabinol is also a naturally occurring component of Cannabis sativa L. (Marijuana). Dronabinol is a light yellow resinous oil that is sticky at room temperature and hardens upon refrigeration. Dronabinol is insoluble in water and is formulated in sesame oil. It has a pKa of 10.6 and an octanol-water partition coefficient: 6,000:1 at pH 7. Capsules for oral administration: Dronabinol capsules are supplied as round, soft gelatin capsules containing either 2.5 mg, 5 mg, or 10 mg dronabinol. Each dronabinol capsule strength is formulated with the following inactive ingredients: 2.5 mg capsule contains gelatin, glycerin, sesame oil, and titanium dioxide; 5 mg capsule contains iron oxide red and iron oxide black, gelatin, glycerin, sesame oil, and titanium dioxide; 10 mg capsule contains iron oxide red and iron oxide yellow, gelatin, glycerin, sesame oil, and titanium dioxide. CLINICAL PHARMACOLOGY Dronabinol is an orally active cannabinoid which, like other cannabinoids, has complex effects on the central nervous system (CNS), including central sympathomimetic activity. Cannabinoid receptors have been discovered in neural tissues. These receptors may play a role in mediating the effects of dronabinol and other cannabinoids. -

Drug Repurposing for the Management of Depression: Where Do We Stand Currently?
life Review Drug Repurposing for the Management of Depression: Where Do We Stand Currently? Hosna Mohammad Sadeghi 1,†, Ida Adeli 1,† , Taraneh Mousavi 1,2, Marzieh Daniali 1,2, Shekoufeh Nikfar 3,4,5 and Mohammad Abdollahi 1,2,* 1 Toxicology and Diseases Group (TDG), Pharmaceutical Sciences Research Center (PSRC), The Institute of Pharmaceutical Sciences (TIPS), Tehran University of Medical Sciences, Tehran 1417614411, Iran; [email protected] (H.M.S.); [email protected] (I.A.); [email protected] (T.M.); [email protected] (M.D.) 2 Department of Toxicology and Pharmacology, School of Pharmacy, Tehran University of Medical Sciences, Tehran 1417614411, Iran 3 Personalized Medicine Research Center, Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences, Tehran 1417614411, Iran; [email protected] 4 Pharmaceutical Sciences Research Center (PSRC) and the Pharmaceutical Management and Economics Research Center (PMERC), Evidence-Based Evaluation of Cost-Effectiveness and Clinical Outcomes Group, The Institute of Pharmaceutical Sciences (TIPS), Tehran University of Medical Sciences, Tehran 1417614411, Iran 5 Department of Pharmacoeconomics and Pharmaceutical Administration, School of Pharmacy, Tehran University of Medical Sciences, Tehran 1417614411, Iran * Correspondence: [email protected] † Equally contributed as first authors. Citation: Mohammad Sadeghi, H.; Abstract: A slow rate of new drug discovery and higher costs of new drug development attracted Adeli, I.; Mousavi, T.; Daniali, M.; the attention of scientists and physicians for the repurposing and repositioning of old medications. Nikfar, S.; Abdollahi, M. Drug Experimental studies and off-label use of drugs have helped drive data for further studies of ap- Repurposing for the Management of proving these medications. -

The Rise and Decline of Cannabis Prohibition the History of Cannabis in the UN Drug Control System and Options for Reform
TRANSNATIONAL I N S T I T U T E THE RISE AND DECLINE OF CANNABIS PROHIBITION THE HISTORY OF CANNABIS IN THE UN DruG CONTROL SYSTEM AND OPTIONS FOR REFORM 3 The Rise and Decline of Cannabis Prohibition Authors Dave Bewley-Taylor Tom Blickman Martin Jelsma Copy editor David Aronson Design Guido Jelsma www.guidojelsma.nl Photo credits Hash Marihuana & Hemp Museum, Amsterdam/ Barcelona Floris Leeuwenberg Pien Metaal UNOG Library/League of Nations Archives UN Photo Printing Jubels, Amsterdam Contact Transnational Institute (TNI) De Wittenstraat 25 1052 AK Amsterdam Netherlands Tel: +31-(0)20-6626608 Fax: +31-(0)20-6757176 [email protected] www.tni.org/drugs www.undrugcontrol.info www.druglawreform.info Global Drug Policy Observatory (GDPO) Research Institute for Arts and Humanities Rooms 201-202 James Callaghan Building Swansea University Financial contributions Singleton Park, Swansea SA2 8PP Tel: +44-(0)1792-604293 This report has been produced with the financial www.swansea.ac.uk/gdpo assistance of the Hash Marihuana & Hemp Museum, twitter: @gdpo_swan Amsterdam/Barcelona, the Open Society Foundations and the Drug Prevention and Information Programme This is an Open Access publication distributed under (DPIP) of the European Union. the terms of the Creative Commons Attribution License The contents of this publication are the sole responsibility (http://creativecommons.org/licenses/by/2.0), which of TNI and GDPO and can under no circumstances be permits unrestricted use, distribution, and reproduction regarded as reflecting the position of the donors. in any medium, provided the original work is properly cited. TNI would appreciate receiving a copy of the text in which this document is used or cited. -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -
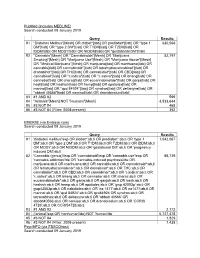
Diabetes Mellitus
PubMed (includes MEDLINE) Search conducted 09 January 2019 Query Results #1 "Diabetes Mellitus"[Mesh] OR diabet*[tiab] OR prediabet*[tiab] OR "type 1 630,566 DM"[tiab] OR "type 2 DM"[tiab] OR T1DM[tiab] OR T2DM[tiab] OR IDDM[tiab] OR MODY[tiab] OR NIDDM[tiab] OR "gestational DM"[tiab] #2 "Cannabis"[Mesh] OR "Cannabinoids"[Mesh] OR "Marijuana 52,197 Smoking"[Mesh] OR "Marijuana Use"[Mesh] OR "Marijuana Abuse"[Mesh] OR "Medical Marijuana"[Mesh] OR marijuana[tiab] OR marihuana[tiab] OR cannabis[tiab] OR cannabinoid*[tiab] OR tetrahydrocannabinol*[tiab] OR dronabinol*[tiab] OR THC[tiab] OR cannabidiol*[tiab] OR CBD[tiab] OR cannabinol*[tiab] OR "c.indica"[tiab] OR "c.sativa"[tiab] OR bhang[tiab] OR cannador[tiab] OR charas[tiab] OR eucannabinolide*[tiab] OR ganja[tiab] OR hash[tiab] OR hashish[tiab] OR hemp[tiab] OR epidiolex[tiab] OR marinol[tiab] OR "qcd 84924"[tiab] OR syndros[tiab] OR deltanyne[tiab] OR "abbott 40566"[tiab] OR namisol[tiab] OR dronabinolum[tiab] #3 #1 AND #2 599 #4 "Animals"[Mesh] NOT "Humans"[Mesh] 4,533,644 #5 #3 NOT #4 468 #6 #3 NOT #4 (Filter: 2008-present) 352 EMBASE (via Embase.com) Search conducted 09 January 2019 Query Results #1 'diabetes mellitus'/exp OR diabet*:ab,ti OR prediabet*:ab,ti OR 'type 1 1,042,067 DM':ab,ti OR 'type 2 DM':ab,ti OR T1DM:ab,ti OR T2DM:ab,ti OR IDDM:ab,ti OR MODY:ab,ti OR NIDDM:ab,ti OR 'gestational DM':ab,ti OR 'pregnancy induced DM':ab,ti #2 'Cannabis (genus)'/exp OR 'cannabinoid'/exp OR 'cannabis use'/exp OR 88,739 'cannabis addiction'/de OR 'cannabis-induced psychosis'/de OR marijuana:ab,ti -

Current Update in the Management of Post-Operative Neuraxial Opioid-Induced Pruritus Borja Mugabure Bujedo
ORIGINAL ARTICLE Current Update in the Management of Post-operative Neuraxial Opioid-induced Pruritus Borja Mugabure Bujedo Acute and Chronic Pain Unit, Department of Anesthesiology, Donostia University Hospital ABSTRACT Introduction: Itching is an extremely bothersome side effect that frequently appears after the epidural and intrathecal administration of opioids. This common side effect can be severe enough to be considered by the patient to be as bad or worse than the pain itself. Both prevention and treatments remain a challenge in the clinical management of these patients. Many drugs have been used to either treat or prevent the condition with variable results. Materials and Methods: This article’s purpose is to review the clinical literature and summarize the current evidence of efficacy and efficiency of pharmacological treatments available to manage opioid-induced pruritus mediated by spinal opioids in the post-operative setting. An analysis of how the mechanism of action of the most common drugs affects in their clinic usefulness is presented. This comprehensive review is limited to published papers in English, found on PubMed, Medline, and Scopus until December 2017. The papers used in this review were systematized reviews, randomized controlled trials, and opinion articles by subject experts. Results: The most useful drugs are mu opioid antagonists, such as naloxone, and mixed opioids kappa agonists/mu antagonists, such as nalbuphine and butorphanol, the latter being capable of maintaining analgesia in addition to reduce itching. Some efficacy has also been observed, to a lesser extent, from 5-hydroxytryptamine 3 serotonin receptor antagonists, such as prophylactically administered ondansetron, and D2 dopaminergic receptor antagonists, such as droperidol. -

Marijuana in Oregon: a Primer for Clinicians
Legalized Marijuana in Oregon: A Primer for Clinicians Katrina Hedberg, MD, MPH Health Officer & State Epidemiologist History • Originated Central Asia • 3000 BC: Chinese Emperor Shen Nung • Ancient Greeks and Romans familiar with cannabis • 1840’s: Dr. WB O’Shaughnessy, a surgeon working for the British East India Company • 1800’s: Hemp grown in US by President Washington • 1937: US marijuana tax law making it illegal Oregon Public Health Division 2 Marijuana: US Legal Status US DEA Controlled Substances Act Schedule 1 • High potential for abuse • No currently accepted medical use in US (federal level); clinical trials lacking 2018: State Laws • 30 States Medical MJ • 9 recreational Oregon Public Health Division 3 Oregon Medical Marijuana Act • December 1998: Oregon voters approved Ballot Measure 67 • Allows medical use of marijuana (for specific conditions) • Established a state-controlled permit system (the OMMP) • Protects patients and doctors from penalties Oregon Public Health Division 4 Marijuana Sales in Oregon • Jan 2014: Oregon Legislature approved Medical Marijuana Dispensaries (OHA regulates) • July 2015: recreational marijuana became legal • Oct 2015: early sales of retail marijuana allowed at medical dispensaries • Oct 2016: retail marijuana stores open (OLCC regulates) Oregon Public Health Division 5 Objectives • Be familiar with cannabis, cannabinoids, and medical conditions for which scientific evidence supports effectiveness. • Describe elements of Oregon’s clinical guidelines for physicians when recommending use of medical -

Drug-Induced Pruritus: a Review
Acta Derm Venereol 2009; 89: 236–244 REVIEW ARTICLE Drug-induced Pruritus: A Review Adam REICH1, Sonja STÄNDER2 and Jacek C. SZepietowsKI1,3 1Department of Dermatology, Venereology and Allergology, Wroclaw Medical University, 2Clinical Neurodermatology, Department of Dermatology, University Hospital of Münster, Germany, and 3Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wroclaw, Poland Pruritus is an unpleasant sensation that leads to scrat- polate to drugs that are prescribed mainly in outpatient ching. In addition to several diseases, the administration clinics, as only inpatients were analysed. In another of drugs may induce pruritus. It is estimated that pru- study on skin reactions due to antibacterial agents used ritus accounts for approximately 5% of all skin adverse in 13,679 patients treated by general practitioners, reactions after drug intake. However, to date there has cutaneous adverse effects were reported in 135 (1%) been no systematic review of the natural course and pos- subjects, and general pruritus accounted for 13.3% of sible underlying mechanisms of drug-induced pruritus. these reactions (4). In a recent analysis of 200 patients For example, no clear distinction has been made between with drug reactions, 12.5% showed pruritus without acute or chronic (lasting more than 6 weeks) forms of skin lesions (5). However, only a few drugs have been pruritus. This review presents a systematic categoriza- analysed more carefully in relation to pruritus, mainly tion of the different forms of drug-induced pruritus, with antimalarials, opioids, and hydroxyethyl starch (see special emphasis on a therapeutic approach to this side- below). Furthermore, analysing the available data on effect. -

5. Cannabis and Cannabis-Related Substances
Annex 1- Extract from the Report of the 41st Expert Committee on Drug Dependence: Cannabis and cannabis-related substances 5. Cannabis and cannabis-related substances 5.1 Cannabis and cannabis resin In the 1961 Single Convention on Narcotic Drugs, cannabis and cannabis resin are described, respectively, as the flowering or fruiting tops of the cannabis plant (excluding the seeds and leaves when not accompanied by the tops) from which the resin has not been extracted and as the separated resin, whether crude or purified, obtained from the cannabis plant. Reference to cannabis below will be taken to also include cannabis resin. Of the many compounds in cannabis, delta-9- tetrahydrocannabinol (Δ9-THC) is the principal psychoactive constituent of cannabis, while cannabidiol (CBD) is also present but is not psychoactive. Following consumption of cannabis, the adverse effects experienced include dizziness and impairment of motor control and cognitive function. As a result of the effects on movement and cognition, cannabis use can impair driving. There are particular risks of cannabis use reported for children, such as respiratory depression, tachycardia and coma. The adverse effects of cannabis consumption are similar to those produced by Δ9-THC alone. There are also a number of adverse effects associated with long term cannabis use, particularly increased risk of mental health disorders such as anxiety, depression and psychotic illness. Chronic regular cannabis use is particularly problematic for young people because of its effects on the developing brain. Cannabis can cause physical dependence in people who use the drug daily or near daily. This is evidenced by the onset of cannabis withdrawal symptoms that occur upon abstinence; these symptoms include gastrointestinal disturbance, appetite changes, irritability, restlessness and sleep impairment.