Orycteropus Aler
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
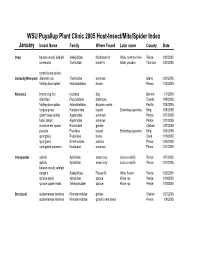
Host Insect List 2005
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

Isoptera Book Chapter
Isoptera 535 See Also the Following Articles Biodiversity ■ Biogeographical Patterns ■ Cave Insects ■ Introduced Insects Further Reading Carlquist , S. ( 1974 ) . “ Island Biology . ” Columbia University Press , New York and London . Gillespie , R. G. , and Roderick , G. K. ( 2002 ) . Arthropods on islands: Colonization, speciation, and conservation . Annu. Rev. Entomol. 47 , 595 – 632 . Gillespie , R. G. , and Clague , D. A. (eds.) (2009 ) . “ Encyclopedia of Islands. ” University of California Press , Berkeley, CA . Howarth , F. G. , and Mull , W. P. ( 1992 ) . “ Hawaiian Insects and Their Kin . ” University of Hawaii Press , Honolulu, HI . MacArthur , R. H. , and Wilson , E. O. ( 1967 ) . “ The Theory of Island Biogeography . ” Princeton University Press , Princeton, NJ . Wagner , W. L. , and Funk , V. (eds.) ( 1995 ) . “ Hawaiian Biogeography Evolution on a Hot Spot Archipelago. ” Smithsonian Institution Press , Washington, DC . Whittaker , R. J. , and Fern á ndez-Palacios , J. M. ( 2007 ) . “ Island Biogeography: Ecology, Evolution, and Conservation , ” 2nd ed. Oxford University Press , Oxford, U.K . I Isoptera (Termites) Vernard R. Lewis FIGURE 1 Castes for Isoptera. A lower termite group, University of California, Berkeley Reticulitermes, is represented. A large queen is depicted in the center. A king is to the left of the queen. A worker and soldier are he ordinal name Isoptera is of Greek origin and refers to below. (Adapted, with permission from Aventis Environmental the two pairs of straight and very similar wings that termites Science, from The Mallis Handbook of Pest Control, 1997.) Thave as reproductive adults. Termites are small and white to tan or sometimes black. They are sometimes called “ white ants ” and can be confused with true ants (Hymenoptera). -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -

James K. Wetterer
James K. Wetterer Wilkes Honors College, Florida Atlantic University 5353 Parkside Drive, Jupiter, FL 33458 Phone: (561) 799-8648; FAX: (561) 799-8602; e-mail: [email protected] EDUCATION UNIVERSITY OF WASHINGTON, Seattle, WA, 9/83 - 8/88 Ph.D., Zoology: Ecology and Evolution; Advisor: Gordon H. Orians. MICHIGAN STATE UNIVERSITY, East Lansing, MI, 9/81 - 9/83 M.S., Zoology: Ecology; Advisors: Earl E. Werner and Donald J. Hall. CORNELL UNIVERSITY, Ithaca, NY, 9/76 - 5/79 A.B., Biology: Ecology and Systematics. UNIVERSITÉ DE PARIS III, France, 1/78 - 5/78 Semester abroad: courses in theater, literature, and history of art. WORK EXPERIENCE FLORIDA ATLANTIC UNIVERSITY, Wilkes Honors College 8/04 - present: Professor 7/98 - 7/04: Associate Professor Teaching: Biodiversity, Principles of Ecology, Behavioral Ecology, Human Ecology, Environmental Studies, Tropical Ecology, Field Biology, Life Science, and Scientific Writing 9/03 - 1/04 & 5/04 - 8/04: Fulbright Scholar; Ants of Trinidad and Tobago COLUMBIA UNIVERSITY, Department of Earth and Environmental Science 7/96 - 6/98: Assistant Professor Teaching: Community Ecology, Behavioral Ecology, and Tropical Ecology WHEATON COLLEGE, Department of Biology 8/94 - 6/96: Visiting Assistant Professor Teaching: General Ecology and Introductory Biology HARVARD UNIVERSITY, Museum of Comparative Zoology 8/91- 6/94: Post-doctoral Fellow; Behavior, ecology, and evolution of fungus-growing ants Advisors: Edward O. Wilson, Naomi Pierce, and Richard Lewontin 9/95 - 1/96: Teaching: Ethology PRINCETON UNIVERSITY, Department of Ecology and Evolutionary Biology 7/89 - 7/91: Research Associate; Ecology and evolution of leaf-cutting ants Advisor: Stephen Hubbell 1/91 - 5/91: Teaching: Tropical Ecology, Introduction to the Scientific Method VANDERBILT UNIVERSITY, Department of Psychology 9/88 - 7/89: Post-doctoral Fellow; Visual psychophysics of fish and horseshoe crabs Advisor: Maureen K. -

Sultan Qaboos University Journal for Scientific Research
Agricultural and Marine Sciences, 10(1):33-40 (2005) ©2005 Sultan Qaboos University Identification, Geographical Distribution and Hosts of Subterranean Termites in the United Arab Emirates Arid Ecosystem W. Kaakeh Department of Arid Land Agriculture, College of Food Systems, P. O. Box 17555, United Arab Emirates University, Al-Ain, United Arab Emirates وﻟﯿﺪ ﻛﻌﻚ اﻟﺨﻼﺻﺔ: ﺗﻢ ﺗﻌﺮﻳﻒ ﺳﺘﺔ أﻧﻮاع ﻣﻦ اﻟﻨﻤﻞ اﻷﺑﯿﺾ (اﻷرﺿﺔ) ﺗﺎﺑﻌﺔ إﻟﻰ ﺧﻤﺴﺔ أﺟﻨﺎس وﺛﻼث ﻓﺼﺎﺋﻞ (ھﻮدوjرﻣﯿﺘﯿﺪي Hodotermitidae، راﻳﻨﻮﺗﺮﻣﯿﺘﯿﺪي Rhinotermi،Rhinotermitidaetidae، وﺗﺮﻣﯿﺘﯿﺪي T(Termitidaeermitidae) ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة. وأﻧﻮاع اﻷرﺿﺔ اﻟﺘﻲ ﺗﻢ ﺗﺴﺠﯿﻠﮭﺎ ھﻲ اﻷرﺿﺔ اﻟﺤﺎﺻﺪة أو اﻷرﺿﺔ اﻟﻼﺷﻮﻛﯿﺔ Anacanthotermes ochraceusochraceus (Burmeister(Burmeister) و Anacanthotermes ubachi (Navas(Navas)، وأرﺿﺔ اﻟﺮﻣﻞ اﻟﺜﻐﺮﻳﺔ Psammotermes hypostomahypostoma (Desneux)، واﻷرﺿﺔ اﻟﺸﻤﻌﯿﺔ اﻟﺼﻐﯿﺮة MicrocerotermesMicrocerotermes diversusdiversus Silvestri))، واﻷرﺿﺔ اﻟﻨﺠﺪﻳﺔ اﻟﺪﻗﯿﻘﺔ Microtermes najdensis (Harris) ، واﻷرﺿﺔ Heterotermes aethiopicus (Sjostedt)، وﺑﺎﺳﺘﺜﻨﺎء اﻟﻨﻮع H. aethiopicus، ﻓﺈﻧﻪ ﺗﻢ ﺗﺴﺠﯿﻞ اﻷﻧﻮاع اﻟﺨﻤﺴﺔ اﻷﺧﺮى ﻟﻠﻤﺮة اﻷوﻟﻰ ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة. وﺗﻌﯿﺶ ﻛﻞ اﻷﻧﻮاع ﺗﺤﺖ اﻷرض وﺗﺼﻞ إﻟﻰ ﻣﺼﺎدر اﻟﻐﺬاء اﻟﺨﺸﺒﯿﺔ ﻣﻦ ﺧﻼل أﻧﻈﻤﺔ اﻷﻧﻔﺎق اﻟﻄﯿﻨﯿﺔ. وﻗﺪ وﺟﺪت اﻷرﺿﺔ ﻓﻲ ﻣﻨﺎﻃﻖ ﻣﺨﺘﻠﻔﺔ ﻣﻦ اﻟﺪوﻟﺔ واﻟﺘﻲ ﺗﺘﻤﯿﺰ ﺑﺎﺧﺘﻼف ﻇﺮوﻓﮭﺎ اﻟﻤﻨﺎﺧﯿﺔ وﻏﻄﺎءھﺎ اﻟﻨﺒﺎﺗﻲ وﻧﻮع ﺗﺮﺑﺘﮭﺎ. وﺗﻔﻀﻞ اﻷرﺿﺔ اﻟﺘﻐﺬﻳﺔ ﻋﻠﻰ اﻟﻌﻮاﺋﻞ اﻟﺤﯿﺔ أو اﻟﻤﯿﺘﺔ أو اﻟﻤﺘﻌﻔﻨﺔ، ﺑﺎﻹﺿﺎﻓﺔ إﻟﻰ اﻟﻤﻮاد ﻏﯿﺮ اﻟﺴﯿﻠﯿﻠﻮزﻳﺔ. وﻣﻦ أﻛﺜﺮ أﻧﻮاع اﻷرﺿﺔ ًﺗﻮزﻳﻌﺎ ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة ھﻲ A. ochraceusochraceus وﺗﺘﺒﻌﮭﺎ ﻛﻞ ﻣﻦ P.P. hypostomahypostoma وdiversusوM. diversus. وﻗﺪ اﺧﺘﻠﻒ ﺗﻮزﻳﻊ اﻷﻧﻮاع اﻟﺴﺘﺔ ﺿﻤﻦ -

Namibia, 2018
Nambia and little bits of Botswana, Zimbabwe, and Zambia, July-August 2018 Michael Kessler In 1994, my wife Elke and I did our first joint trip to Namibia, spending 3 weeks mainly in the arid western parts of the country and seeing such goodies as Brown Hyena, Caracal, Black Mongoose, Honey Badger, 3 species of sengis, and Southern African Porcupine. In 2010, we made our first family trip to Africa to KwaZulu Natal, seeing much of the large game. So now we decided to return to Namibia with the family, with the aim of exploring some new areas and searching out the less easily seen species. Time and budget limited the trip to about 2½ weeks and after some deliberation, we settled on the following sites: Sesriem + Sossusvlei for the dunes; Walvisbay for Heaviside’s Dolphin + Welwitschia; Erindi for African Wild Dog and the other game; Toko Lodge for the night drives, especially for Aardvark; Etosha only briefly for the amazing wildlife spectacle; Mahango for the Okavango specials (birds and mammals); and Victoria Falls for, well, the falls. This resulted in the following Itinerary: 25.7: Left Zurich in the evening, arriving on 26.7.: am in Jo’burg, followed by a connecting flight to Windhoek where we picked up the rental car, did some grocery shopping, and fell into our beds at Arebbusch Lodge on the southern outskirts of the city. 27.7.: Long drive to Desert Homestead Lodge at Sesriem. 28.7.: am: visit to Sossusvlei; pm: Cessna flight over the dunes, followed by a night drive back to the lodge, seeing some Bat-eared Foxes. -

Stomach Contents of the Indian Pangolin Manis Crassicaudata (Mammalia: Pholidota: Manidae) in Tropical Forests of Southern India
OPEN ACCESS The Journal of Threatened Taxa fs dedfcated to bufldfng evfdence for conservafon globally by publfshfng peer-revfewed arfcles onlfne every month at a reasonably rapfd rate at www.threatenedtaxa.org . All arfcles publfshed fn JoTT are regfstered under Creafve Commons Atrfbufon 4.0 Internafonal Lfcense unless otherwfse menfoned. JoTT allows unrestrfcted use of arfcles fn any medfum, reproducfon, and dfstrfbufon by provfdfng adequate credft to the authors and the source of publfcafon. Journal of Threatened Taxa Bufldfng evfdence for conservafon globally www.threatenedtaxa.org ISSN 0974-7907 (Onlfne) | ISSN 0974-7893 (Prfnt) Note Stomach contents of the Indfan Pangolfn Manfs crassfcaudata (Mammalfa: Pholfdota: Manfdae) fn tropfcal forests of southern Indfa Mohanarangan Ashokkumar, Dfpfka Valsarajan, M. Arjun Suresh, Anuraj R. Kafmal & George Chandy 26 May 2017 | Vol. 9| No. 5 | Pp. 10246–10248 10.11609/jot. 2873 .9. 5.10246-10248 For Focus, Scope, Afms, Polfcfes and Gufdelfnes vfsft htp://threatenedtaxa.org/About_JoTT For Arfcle Submfssfon Gufdelfnes vfsft htp://threatenedtaxa.org/Submfssfon_Gufdelfnes For Polfcfes agafnst Scfenffc Mfsconduct vfsft htp://threatenedtaxa.org/JoTT_Polfcy_agafnst_Scfenffc_Mfsconduct For reprfnts contact <[email protected]> Publfsher/Host Partner Threatened Taxa Journal of Threatened Taxa | www.threatenedtaxa.org | 26 May 2017 | 9(5): 10246–10248 Note Pangolins are scaly anteaters and Stomach contents of the are unique in being the world’s only Indian Pangolin Manis crassicaudata true scaly mammals, distributed in (Mammalia: Pholidota: Manidae) ISSN 0974-7907 (Online) Asia and Africa. Of the eight species in tropical forests of southern India ISSN 0974-7893 (Print) of pangolin, four species are found in Asia, the Chinese Pangolin Manis Mohanarangan Ashokkumar 1, Dipika Valsarajan 2, OPEN ACCESS pentadactyla, Indian Pangolin M. -

Twin Mammals and COVID-19: Life and Science of the Suspects
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 27 March 2020 doi:10.20944/preprints202003.0410.v1 Twin Mammals and COVID-19: Life and Science of the Suspects Naser A. Anjum Department of Botany, Aligarh Muslim University, Aligarh – 202 002 U.P. (India) e-mail: [email protected]; [email protected] SCOPUS author ID: https://www.scopus.com/authid/detail.uri?authorId=23097123400 © 2020 by the author(s). Distributed under a Creative Commons CC BY license. Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 27 March 2020 doi:10.20944/preprints202003.0410.v1 Abstract The world is puzzling over the origin of the current outbreak of the coronavirus disease (COVID-19) that is caused by a novel coronavirus-2019 (2019-nCoV). As of 25th March 2020, the World Health Organization has reported 4,14,179 confirmed cases and 18,440 confirmed deaths in total due to COVID-19. To this end, two unique mammals namely bats and pangolins are being investigated for their potential link to COVID-19. However, the evidence so far gathered in this context is far from clear. This paper aimed to: (i) enlighten the major aspects of life of bats and pangolins; (ii) briefly discusses their potential link to COVID-19; and also (iii) to highlight the way forward. The outcomes may contribute to future research on the subject. Keywords Coronavirus; 2019-nCoV; COVID-19; bat, pangolin Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 27 March 2020 doi:10.20944/preprints202003.0410.v1 1. Introduction In humans, several respiratory infections ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS) are known to be due a large family of viruses called as coronaviruses. -

Smutsia Temminckii – Temminck's Ground Pangolin
Smutsia temminckii – Temminck’s Ground Pangolin African Pangolin Working Group and the IUCN Species Survival Commission Pangolin Specialist Group) is Temminck’s Ground Pangolin. No subspecies are recognised. Assessment Rationale The charismatic and poorly known Temminck’s Ground Pangolin, while widely distributed across the savannah regions of the assessment region, are severely threatened by electrified fences (an estimated 377–1,028 individuals electrocuted / year), local and international bushmeat and traditional medicine trades (since 2010, the number of Darren Pietersen confiscations at ports / year has increased exponentially), road collisions (an estimated 280 killed / year) and incidental mortalities in gin traps. The extent of occurrence Regional Red List status (2016) Vulnerable A4cd*†‡ has been reduced by an estimated 9–48% over 30 years National Red List status (2004) Vulnerable C1 (1985 to 2015), due to presumed local extinction from the Free State, Eastern Cape and much of southern KwaZulu- Reasons for change No change Natal provinces. However, the central interior (Free State Global Red List status (2014) Vulnerable A4d and north-eastern Eastern Cape Province) were certainly never core areas for this species and thus it is likely that TOPS listing (NEMBA) (2007) Vulnerable the corresponding population decline was far lower overall CITES listing (2000) Appendix II than suggested by the loss of EOO. Additionally, rural settlements have expanded by 1–9% between 2000 and Endemic No 2013, which we infer as increasing poaching pressure and *Watch-list Data †Watch-list Threat ‡Conservation Dependent electric fence construction. While throughout the rest of Africa, and Estimated mature population size ranges widely increasingly within the assessment region, local depending on estimates of area of occupancy, from 7,002 and international illegal trade for bushmeat and to 32,135 animals. -

Encyclopedia of Social Insects
G Guests of Social Insects resources and homeostatic conditions. At the same time, successful adaptation to the inner envi- Thomas Parmentier ronment shields them from many predators that Terrestrial Ecology Unit (TEREC), Department of cannot penetrate this hostile space. Social insect Biology, Ghent University, Ghent, Belgium associates are generally known as their guests Laboratory of Socioecology and Socioevolution, or inquilines (Lat. inquilinus: tenant, lodger). KU Leuven, Leuven, Belgium Most such guests live permanently in the host’s Research Unit of Environmental and nest, while some also spend a part of their life Evolutionary Biology, Namur Institute of cycle outside of it. Guests are typically arthropods Complex Systems, and Institute of Life, Earth, associated with one of the four groups of eusocial and the Environment, University of Namur, insects. They are referred to as myrmecophiles Namur, Belgium or ant guests, termitophiles, melittophiles or bee guests, and sphecophiles or wasp guests. The term “myrmecophile” can also be used in a broad sense Synonyms to characterize any organism that depends on ants, including some bacteria, fungi, plants, aphids, Inquilines; Myrmecophiles; Nest parasites; and even birds. It is used here in the narrow Symbionts; Termitophiles sense of arthropods that associated closely with ant nests. Social insect nests may also be parasit- Social insect nests provide a rich microhabitat, ized by other social insects, commonly known as often lavishly endowed with long-lasting social parasites. Although some strategies (mainly resources, such as brood, retrieved or cultivated chemical deception) are similar, the guests of food, and nutrient-rich refuse. Moreover, nest social insects and social parasites greatly differ temperature and humidity are often strictly regu- in terms of their biology, host interaction, host lated. -

Evolution Des Caractères Crâniens Et Endocrâniens Chez Les Afrotheria (Mammalia) Et Phylogénie Du Groupe Julien Benoit
Evolution des caractères crâniens et endocrâniens chez les Afrotheria (Mammalia) et phylogénie du groupe Julien Benoit To cite this version: Julien Benoit. Evolution des caractères crâniens et endocrâniens chez les Afrotheria (Mammalia) et phylogénie du groupe. Biologie animale. Université Montpellier II - Sciences et Techniques du Languedoc, 2013. Français. NNT : 2013MON20073. tel-01001999 HAL Id: tel-01001999 https://tel.archives-ouvertes.fr/tel-01001999 Submitted on 5 Jun 2014 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Thèse Pour l’obtention du grade de DOCTEUR DE L’UNIVERSITE MONTPELLIER II Discipline : Paléontologie Formation Doctorale : Paléontologie, Paléobiologie et Phylogénie Ecole Doctorale : Systèmes Intégrés en Biologie, Agronomie, Géosciences, Hydrosciences, Environnement Présentée et soutenue publiquement par Benoit Julien Le 6 Novembre 2013 Titre : Evolution des caractères crâniens et endocrâniens chez les Afrotheria (Mammalia) et phylogénie du groupe Thèse dirigée par Rodolphe Tabuce et Monique Vianey-Liaud Jury Lecturer and Curator, Dr. Asher Robert Rapporteur University Museum of Zoology, Cambridge Directeur de Recherche au CNRS, Dr. Gheerbrant Emmanuel Rapporteur Muséum d’Histoire Naturelle, Paris Professeur, Pr. Tassy Pascal Examinateur Muséum d’Histoire Naturelle, Paris Coordinateur du groupe de Recherche en Paléomammalogie, Dr. -

Impact of the Invasive Crazy Ant Anoplolepis Gracilipes on Bird Island, Seychelles
Journal of Insect Conservation 8: 15–25, 2004. 15 # 2004 Kluwer Academic Publishers. Printed in the Netherlands. Impact of the invasive crazy ant Anoplolepis gracilipes on Bird Island, Seychelles J. Gerlach University Museum of Zoology Cambridge, Department of Zoology, Downing Street, Cambridge CB2 3EJ, UK (e-mail: [email protected]) Received 26 March 2003; accepted in revised form 12 December 2003 Key words: Anoplolepis gracilipes, Ant, Formicidae, Invasion, Management, Seychelles Abstract The crazy ant (Anoplolepis gracilipes) invaded Bird island, Seychelles, in the 1980s. In 1997, its range expanded and population densities increased. The impacts of this change were studied in 2001 using a combination of arthropod collecting methods. The ant population excluded larger invertebrates (principally the large ant Odontomachus simillimus and the crabs, principally Ocypode spp.). Cockroaches, however, remained abundant in ant-infested areas and tree-nesting birds (Lesser Noddy Anous tenuirostris) appear to be able to breed successfully in the presence of the crazy ant. The ants are only abundant in areas of deep shade which provide cool nesting areas, yet enabling them to forage in the open when ground temperatures fall. The expansion of the ants was correlated with the regeneration of woodland on the island. Recommendations are made for the management of the woodland which may reduce the impacts of the crazy ant. Introduction Africa, Asia, America and Australia (Dorow 1996). This species has been recorded as excluding The threat from alien invasive species is widely other ant species (Greenslade 1971) and recently perceived as one of the major contributions to has been implicated in a population crash in the present-day high-extinction rates, affecting an esti- red crab Gecarcoidea natalis on Christmas Island mated 30% of threatened birds, 15% of threatened (O’ Dowd et al.