Effect of Controlled Lighting on Band-Tailed Pigeon (Patagioenas Fasciata) Breeding
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Band-Tailed Pigeon (Patagioenas Fasciata)
Band-tailed Pigeon (Patagioenas fasciata) NMPIF level: Species Conservation Concern, Level 2 (SC2) NMPIF assessment score: 15 NM stewardship responsibility: Low National PIF status: Watch List New Mexico BCRs: 16, 34, 35 Primary breeding habitat(s): Ponderosa Pine Forest, Mixed Conifer Forest Other habitats used: Spruce-Fir Forest, Madrean Pine-Oak Woodland Summary of Concern Band-tailed Pigeon is a summer resident of montane forests in New Mexico. Both locally and across its wide geographic range the species has shown sharp population declines since the 1960s. Prior to that time, extensive commercial hunting may have significantly reduced the population from historic levels. It is not known if current declines are the result of continuing hunting pressure, habitat changes, or other factors. Associated Species Northern Goshawk, Long-eared Owl, Lewis's Woodpecker (SC1), Acorn Woodpecker, Hairy Woodpecker, Olive-sided Flycatcher (BC2), Greater Pewee (BC2), Steller's Jay, Red-faced Warbler (SC1). Distribution A Pacific coast population of Band-tailed Pigeon breeds from central California north to Canada and Alaska, extending south to Baja California in the winter. In the interior, a migratory population breeds in upland areas of southern Utah, Colorado, Arizona and New Mexico. The species occurs year-round throughout the highlands of central Mexico, south to Central and South America. In New Mexico, Band-tailed Pigeon breeds in forest habitat throughout the state, west of the plains. It is perhaps most common in the southwest (Parmeter et al. 2002). Ecology and Habitat Requirements In the Southwest, Band-tailed Pigeons inhabit montane forests dominated by pines and oaks, sometimes extending upward in elevation to timberline. -

Checklistccamp2016.Pdf
2 3 Participant’s Name: Tour Company: Date#1: / / Tour locations Date #2: / / Tour locations Date #3: / / Tour locations Date #4: / / Tour locations Date #5: / / Tour locations Date #6: / / Tour locations Date #7: / / Tour locations Date #8: / / Tour locations Codes used in Column A Codes Sample Species a = Abundant Red-lored Parrot c = Common White-headed Wren u = Uncommon Gray-cheeked Nunlet r = Rare Sapayoa vr = Very rare Wing-banded Antbird m = Migrant Bay-breasted Warbler x = Accidental Dwarf Cuckoo (E) = Endemic Stripe-cheeked Woodpecker Species marked with an asterisk (*) can be found in the birding areas visited on the tour outside of the immediate Canopy Camp property such as Nusagandi, San Francisco Reserve, El Real and Darien National Park/Cerro Pirre. Of course, 4with incredible biodiversity and changing environments, there is always the possibility to see species not listed here. If you have a sighting not on this list, please let us know! No. Bird Species 1A 2 3 4 5 6 7 8 Tinamous Great Tinamou u 1 Tinamus major Little Tinamou c 2 Crypturellus soui Ducks Black-bellied Whistling-Duck 3 Dendrocygna autumnalis u Muscovy Duck 4 Cairina moschata r Blue-winged Teal 5 Anas discors m Curassows, Guans & Chachalacas Gray-headed Chachalaca 6 Ortalis cinereiceps c Crested Guan 7 Penelope purpurascens u Great Curassow 8 Crax rubra r New World Quails Tawny-faced Quail 9 Rhynchortyx cinctus r* Marbled Wood-Quail 10 Odontophorus gujanensis r* Black-eared Wood-Quail 11 Odontophorus melanotis u Grebes Least Grebe 12 Tachybaptus dominicus u www.canopytower.com 3 BirdChecklist No. -

Pigeon, Band-Tailed
Pigeons and Doves — Family Columbidae 269 Band-tailed Pigeon Patagioenas fasciata California’s only native large pigeon can be seen year round in San Diego’s mountains, sometimes in large flocks, sometimes as only scattered individu- als. Elderberries and acorns are its staple foods, so the Band-tailed Pigeon frequents woodland with abundant oaks. Though it inhabits all the mountains where the black and canyon live oaks are common, its distribution is oddly patchy in foothill woodland dominated by the coast live oak. Both migratory and nomadic, the Band-tailed Pigeon may be common in some areas in some years and absent in others; it shows up occasionally in all regions of the county as Photo by Anthony Mercieca a vagrant. Breeding distribution: As its name in Spanish suggests, ries in dry scrub. Eleanor Beemer noted this movement Palomar Mountain is the center of Band-tailed Pigeon at Pauma Valley in the 1930s, and it continues today. abundance in San Diego County (paloma = pigeon or Some of our larger summer counts, including the largest, dove). The pigeons move up and down the mountain, were in elderberries around the base of Palomar: 35 near descending to the base in summer to feed on elderber- Rincon (F13) 7 July 2000 (M. B. Mosher); 110 in Dameron Valley (C16) 23 June 2001 (K. L. Weaver). Most of the birds nest in the forested area high on the mountain, but some nest around the base, as shown by a fledgling in Pauma Valley (E12) 19 May 2001 (E. C. Hall) and an occupied nest near the West Fork Conservation Camp (E17) 12 May 2001 (J. -

B.Sc. II YEAR CHORDATA
B.Sc. II YEAR CHORDATA CHORDATA 16SCCZO3 Dr. R. JENNI & Dr. R. DHANAPAL DEPARTMENT OF ZOOLOGY M. R. GOVT. ARTS COLLEGE MANNARGUDI CONTENTS CHORDATA COURSE CODE: 16SCCZO3 Block and Unit title Block I (Primitive chordates) 1 Origin of chordates: Introduction and charterers of chordates. Classification of chordates up to order level. 2 Hemichordates: General characters and classification up to order level. Study of Balanoglossus and its affinities. 3 Urochordata: General characters and classification up to order level. Study of Herdmania and its affinities. 4 Cephalochordates: General characters and classification up to order level. Study of Branchiostoma (Amphioxus) and its affinities. 5 Cyclostomata (Agnatha) General characters and classification up to order level. Study of Petromyzon and its affinities. Block II (Lower chordates) 6 Fishes: General characters and classification up to order level. Types of scales and fins of fishes, Scoliodon as type study, migration and parental care in fishes. 7 Amphibians: General characters and classification up to order level, Rana tigrina as type study, parental care, neoteny and paedogenesis. 8 Reptilia: General characters and classification up to order level, extinct reptiles. Uromastix as type study. Identification of poisonous and non-poisonous snakes and biting mechanism of snakes. 9 Aves: General characters and classification up to order level. Study of Columba (Pigeon) and Characters of Archaeopteryx. Flight adaptations & bird migration. 10 Mammalia: General characters and classification up -

Bird) Species List
Aves (Bird) Species List Higher Classification1 Kingdom: Animalia, Phyllum: Chordata, Class: Reptilia, Diapsida, Archosauria, Aves Order (O:) and Family (F:) English Name2 Scientific Name3 O: Tinamiformes (Tinamous) F: Tinamidae (Tinamous) Great Tinamou Tinamus major Highland Tinamou Nothocercus bonapartei O: Galliformes (Turkeys, Pheasants & Quail) F: Cracidae Black Guan Chamaepetes unicolor (Chachalacas, Guans & Curassows) Gray-headed Chachalaca Ortalis cinereiceps F: Odontophoridae (New World Quail) Black-breasted Wood-quail Odontophorus leucolaemus Buffy-crowned Wood-Partridge Dendrortyx leucophrys Marbled Wood-Quail Odontophorus gujanensis Spotted Wood-Quail Odontophorus guttatus O: Suliformes (Cormorants) F: Fregatidae (Frigatebirds) Magnificent Frigatebird Fregata magnificens O: Pelecaniformes (Pelicans, Tropicbirds & Allies) F: Ardeidae (Herons, Egrets & Bitterns) Cattle Egret Bubulcus ibis O: Charadriiformes (Sandpipers & Allies) F: Scolopacidae (Sandpipers) Spotted Sandpiper Actitis macularius O: Gruiformes (Cranes & Allies) F: Rallidae (Rails) Gray-Cowled Wood-Rail Aramides cajaneus O: Accipitriformes (Diurnal Birds of Prey) F: Cathartidae (Vultures & Condors) Black Vulture Coragyps atratus Turkey Vulture Cathartes aura F: Pandionidae (Osprey) Osprey Pandion haliaetus F: Accipitridae (Hawks, Eagles & Kites) Barred Hawk Morphnarchus princeps Broad-winged Hawk Buteo platypterus Double-toothed Kite Harpagus bidentatus Gray-headed Kite Leptodon cayanensis Northern Harrier Circus cyaneus Ornate Hawk-Eagle Spizaetus ornatus Red-tailed -

Alpha Codes for 2168 Bird Species (And 113 Non-Species Taxa) in Accordance with the 62Nd AOU Supplement (2021), Sorted Taxonomically
Four-letter (English Name) and Six-letter (Scientific Name) Alpha Codes for 2168 Bird Species (and 113 Non-Species Taxa) in accordance with the 62nd AOU Supplement (2021), sorted taxonomically Prepared by Peter Pyle and David F. DeSante The Institute for Bird Populations www.birdpop.org ENGLISH NAME 4-LETTER CODE SCIENTIFIC NAME 6-LETTER CODE Highland Tinamou HITI Nothocercus bonapartei NOTBON Great Tinamou GRTI Tinamus major TINMAJ Little Tinamou LITI Crypturellus soui CRYSOU Thicket Tinamou THTI Crypturellus cinnamomeus CRYCIN Slaty-breasted Tinamou SBTI Crypturellus boucardi CRYBOU Choco Tinamou CHTI Crypturellus kerriae CRYKER White-faced Whistling-Duck WFWD Dendrocygna viduata DENVID Black-bellied Whistling-Duck BBWD Dendrocygna autumnalis DENAUT West Indian Whistling-Duck WIWD Dendrocygna arborea DENARB Fulvous Whistling-Duck FUWD Dendrocygna bicolor DENBIC Emperor Goose EMGO Anser canagicus ANSCAN Snow Goose SNGO Anser caerulescens ANSCAE + Lesser Snow Goose White-morph LSGW Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Intermediate-morph LSGI Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Blue-morph LSGB Anser caerulescens caerulescens ANSCCA + Greater Snow Goose White-morph GSGW Anser caerulescens atlantica ANSCAT + Greater Snow Goose Intermediate-morph GSGI Anser caerulescens atlantica ANSCAT + Greater Snow Goose Blue-morph GSGB Anser caerulescens atlantica ANSCAT + Snow X Ross's Goose Hybrid SRGH Anser caerulescens x rossii ANSCAR + Snow/Ross's Goose SRGO Anser caerulescens/rossii ANSCRO Ross's Goose -

Band-Tailed Pigeon, Photo by ©Robert Shantz Size Inches 8; Nests More Likely Where Canopy Closure and Tree Height Are Greater Than Average for the Area 10
Breeding Habitat Use Profile Habitats Used in Arizona Primary: Mixed Conifer Forest Secondary: Pine Forest and Madrean Pine-Oak Woodlands Key Habitat Parameters Plant Composition Mixed conifer and pine-oak, including white fir, Douglas fir, ponderosa pine, Gambel oak; Madrean pine-oak: evergreen oaks, Chihuahua and Apache pines8 Plant Density and 60 – 200 trees/ac 9; typical DBH 6 – 12 Band-tailed Pigeon, photo by ©Robert Shantz Size inches 8; nests more likely where canopy closure and tree height are greater than average for the area 10 Conservation Profile Microhabitat Berry-producing shrubs and oaks enhance Species Concerns Features habitat; but well-developed shrub under- story not required 8 Catastrophic Fires Conservation Status Lists Landscape Wide-ranging or nomadic; may forage long distances from nest; area requirements USFWS 1 No unknown AZGFD 2 Tier 1C Elevation Range in Arizona 3 DoD No 11 BLM 4 No 4,800 – 9,400 feet PIF Watch List 5b Yellow List Density Estimate PIF Regional Concern 5a None Home range: 750 – 450,000 acres (reported in Oregon) 8 Migratory Bird Treaty Act Density: unknown Covered PIF Breeding Population Size Estimates 6 Arizona 40,000 ◑ Natural History Profile Global 6,100,000 ◑ Seasonal Distribution in Arizona Percent in Arizona .65% Breeding Mid-April – August 11; occasionally later PIF Population Goal 5b Migration April – May; September – October Reverse Decline Winter Irregular or absent November – March Trends in Arizona Nest and Nesting Habits Historical (pre-BBS) Unknown 8 Type of Nest Platform 7 BBS -
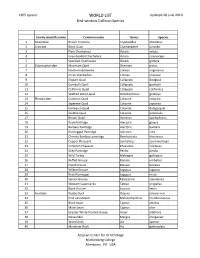
WORLD LIST Updated 28 June 2019 Bird-Window Collision Species
1305 species WORLD LIST Updated 28 June 2019 Bird-window Collision Species Family scientific name Common name Genus Species 1 Tinamidae Brown Tinamou Crypturellus obsoletus 2 Cracidae Black Guan Chamaepetes unicolor 3 Plain Chachalaca Ortalis vetula 4 Grey-headed Chachalaca Ortalis cinereiceps 5 Speckled Chachalaca Ortalis guttata 6 Odontophoridae Mountain Quail Oreortyx pictus 7 Northern Bobwhite Colinus virginianus 8 Crested Bobwhite Colinus cristatus 9 Elegant Quail Callipepla douglasii 10 Gambel's Quail Callipepla gambelii 11 California Quail Callipepla californica 12 Spotted Wood-quail Odontophorus guttatus 13 Phasianidae Common Quail Coturnix coturnix 14 Japanese Quail Coturnix japonica 15 Harlequin Quail Coturnix delegorguei 16 Stubble Quail Coturnix pectoralis 17 Brown Quail Synoicus ypsilophorus 18 Rock Partridge Alectoris graeca 19 Barbary Partridge Alectoris barbara 20 Red-legged Partridge Alectoris rufa 21 Chinese Bamboo-partridge Bambusicola thoracicus 22 Copper Pheasant Syrmaticus soemmerringii 23 Common Pheasant Phasianus colchicus 24 Grey Partridge Perdix perdix 25 Wild Turkey Meleagris gallopavo 26 Ruffed Grouse Bonasa umbellus 27 Hazel Grouse Bonasa bonasia 28 Willow Grouse Lagopus lagopus 29 Rock Ptarmigan Lagopus muta 30 Spruce Grouse Falcipennis canadensis 31 Western Capercaillie Tetrao urogallus 32 Black Grouse Lyrurus tetrix 33 Anatidae Ruddy Duck Oxyura jamaicensis 34 Pink-eared Duck Malacorhynchus membranaceus 35 Black Swan Cygnus atratus 36 Mute Swan Cygnus olor 37 Greater White-fronted Goose Anser albifrons 38 -

UNC-11, a Caenorhabditis Elegans AP180 Homologue, Regulates The
The Auk 118(4):874-887, 2001 A MOLECULAR PHYLOGENY OF THE DOVE GENERA STREPTOPELIA AND COLUMBA K ev in P. Jo h n so n ,1-3-5 S elv in o de K o r t ,2 K a ren D in w o o d ey ,3 A. C. M a tem a n ,4 C a r el ten C a t e ,2 C. M . L essells,4 a n d D a le H. C la y to n 3 1Illinois Natural History Survey, Champaign, Illinois 61820, USA; institute of Evolutionary and Ecological Sciences, Leiden University, Leiden, The Netherlands; departm en t o f Biology, University o f Utah, Salt Lake City, Utah 84112, USA; and Netherlands Institute of Ecology, Heteren, The Netherlands A b s t r a c t . — Evolutionary history of the dove genus Streptopelia has not been examined with rigorous phylogenetic methods. We present a study of phylogenetic relationships of Streptopelia based on over 3,600 base pairs of nuclear and mitochondrial gene sequences. To test for monophyly of Streptopelia, we used several other columbiform taxa, including Colum- ba (Old and New World), Macropygia, Reinwardtoena, and the enigmatic Pink Pigeon (Nesoenas mayeri). On the basis of our analyses, Streptopelia (as currently defined) is not monophyletic; Nesoenas mayeri is the sister species to S. picturata, resulting in paraphyly of Streptopelia. Three main clades of Streptopelia are identified: (1) S. chinensis plus S. senegalensis, (2) S. picturata plus Nesoenas mayeri, and (3) all other species of Streptopelia. It is unclear whether those clades form a monophyletic group to the exclusion of Old World Columba, but several analyses pro duce that result. -

The Pigeon Names Columba Livia, ‘C
Thomas M. Donegan 14 Bull. B.O.C. 2016 136(1) The pigeon names Columba livia, ‘C. domestica’ and C. oenas and their type specimens by Thomas M. Donegan Received 16 March 2015 Summary.—The name Columba domestica Linnaeus, 1758, is senior to Columba livia J. F. Gmelin, 1789, but both names apply to the same biological species, Rock Dove or Feral Pigeon, which is widely known as C. livia. The type series of livia is mixed, including specimens of Stock Dove C. oenas, wild Rock Dove, various domestic pigeon breeds and two other pigeon species that are not congeners. In the absence of a plate unambiguously depicting a wild bird being cited in the original description, a neotype for livia is designated based on a Fair Isle (Scotland) specimen. The name domestica is based on specimens of the ‘runt’ breed, originally illustrated by Aldrovandi (1600) and copied by Willughby (1678) and a female domestic specimen studied but not illustrated by the latter. The name C. oenas Linnaeus, 1758, is also based on a mixed series, including at least one Feral Pigeon. The individual illustrated in one of Aldrovandi’s (1600) oenas plates is designated as a lectotype, type locality Bologna, Italy. The names Columba gutturosa Linnaeus, 1758, and Columba cucullata Linnaeus, 1758, cannot be suppressed given their limited usage. The issue of priority between livia and domestica, and between both of them and gutturosa and cucullata, requires ICZN attention. Other names introduced by Linnaeus (1758) or Gmelin (1789) based on domestic breeds are considered invalid, subject to implicit first reviser actions or nomina oblita with respect to livia and domestica. -

Patagioenas Fasciata (Band-Tailed Pigeon)
UWI The Online Guide to the Animals of Trinidad and Tobago Ecology Patagioenas fasciata (Band-tailed Pigeon) Family: Columbidae (Pigeons and Doves) Order: Columbiformes (Pigeons, Doves and Dodos) Class: Aves (Birds) Fig. 1. Band-tailed pigeon, Patagioenas fasciata. [http://www.wildlifenorthamerica.com/Bird/Band-tailed-Pigeon/Patagioenas/fasciata.html, downloaded 15 February 2017] TRAITS. The band-tailed pigeon is a heavily built bird 30-40cm in length. Both males and females weigh up to 340g (Hammon, 2001). The adult male is dark grey, has a distinct long, fanlike tail with a broad black band. The grey is darker on the wings, with a mauve tint on the breast and a white collar at the nape of the neck, above a shimmery green layer on the lower neck (Fig. 1) (Restall et al., 2006). The eyes are black, surrounded by red eye-ring, and the bill, legs and feet are yellow. Females are like males, but duller and less iridescent, and the tail is virtually unbanded (Hammon, 2001). DISTRIBUTION. Band-tailed pigeons are widely distributed in distinct regions of the Americas (Fig. 2). In the summer breeding season they inhabit dry mountain forest of four states in the southwestern United States, and wet forested areas of the Pacific Coast region (Keppie and Braun, 2000a). They are also year-round residents of Central America and parts of South America, UWI The Online Guide to the Animals of Trinidad and Tobago Ecology extending to the south of Argentina (Hammon, 2001). It is locally common but decreasing in Colombia and Venezuela, and is now a rare visitor to Trinidad (with no records in Tobago). -

Status and Occurrence of Oriental Turtle-Dove (Streptopelia Orientalis) in British Columbia
Status and Occurrence of Oriental Turtle-Dove (Streptopelia orientalis) in British Columbia. By Rick Toochin. Submitted: April 15, 2017. Introduction and Distribution The Oriental Turtle-Dove (Streptopelia orientalis) is a large Dove species that is mostly sedentary in much of its range, but the northern subspecies are migratory (Brazil 2009). This species is found as a permanent resident in South and Central Asia from India, north to north- western Pakistan, north-western Afghanistan, north into Tajikistan, western Kyrgyzstan, northern Kazakhstan, across southern areas of Siberia, Mongolia, through most of China, Korea, and southern Japan, south to Vietnam, throughout South-east Asia to Malaysia, north to Burma and Bangladesh (Clements et al. 2015). The breeding range of the migratory subspecies of the Oriental Turtle-Dove is as far west as the Ural Mountains and includes northern Kazakhstan, across Siberia, throughout northern Mongolia, to Sakhalin Island and northern Japan (Brazil 2009). There are 6 recognized subspecies of the Oriental Turtle-Dove (Clements et al. 2015). Some are migratory and some have restricted ranges. The nominate subspecies of Oriental Turtle-Dove (Streptopelia orientalis orientalis) is highly migratory and is found breeding in central Siberia to China, Korea, Japan and Kuril Islands and accounts for all North American records from Alaska to California (Gibson and Kessel 1997, Hamilton et al. 2007, Brazil 2009, Clements et al. 2015). The second subspecies of Oriental Turtle-Dove (Streptopelia orientalis meena) is also migratory and is found from the Ural Mountains of northern Kazakhstan, through south-western Siberia to Iran, Afghanistan, Kashmir and Nepal (Goodwin 1983, Gibbs et al.