EC Certificate - Production Quality Assurance Directive 93/42/EEC on Medical Devices, Annex V
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geriatric Medicine
Programme Programme identifier – F1 Trust Site F1-1 F1-2 F1-3 Programme identifier – F2 Trust Site F2-1 F2-2 F2-3 Type Barking, Havering and Redbridge Gastroenterology - (Medicine) [Acute] {KING GEORGE Geriatric Medicine - (Medicine) [Acute] {KING GEORGE General Surgery - (Surgery) [Acute] {KING GEORGE NEWHAM GENERAL HOSPITAL Emergency Medicine - (Other) [Acute] {NEWHAM Obstetrics and Gynaecology - (Other) [Acute] {NEWHAM Cardiology - (Medicine) [Acute] {NEWHAM GENERAL Standard 20/LDN/BHRKG/F1/001 KING GEORGE HOSPITAL (RF4DG) 21/LDN/BHNEW/F2/007 BARTS HEALTH NHS TRUST University Hospitals NHS Trust HOSPITAL (RF4DG) LDN/RF4DG/FND/FY1/018} HOSPITAL (RF4DG) LDN/RF4DG/FND/FY1/006} HOSPITAL (RF4DG) LDN/RF4DG/FND/FY1/003} (R1HNH) GENERAL HOSPITAL (R1HNH) LDN/R1HNH/FND/FY2/012} GENERAL HOSPITAL (R1HNH) LDN/R1HNH/FND/FY2/025} HOSPITAL (R1HNH) LDN/R1HNH/FND/FY2/005} Geriatric Medicine - (Medicine) [Acute] {HOMERTON Emergency Medicine - (Other) [Acute] {HOMERTON Barking, Havering and Redbridge General Surgery - (Surgery) [Acute] {KING GEORGE Gastroenterology - (Medicine) [Acute] {KING GEORGE Geriatric Medicine - (Medicine) [Acute] {KING GEORGE HOMERTON UNIVERSITY HOSPITAL HOMERTON UNIVERSITY General Practice - (Other) [Community] {Homerton - Standard 20/LDN/BHRKG/F1/002 KING GEORGE HOSPITAL (RF4DG) 21/LDN/HOMFT/F2/021 UNIVERSITY HOSPITAL (RQXM1) UNIVERSITY HOSPITAL (RQXM1) University Hospitals NHS Trust HOSPITAL (RF4DG) LDN/RF4DG/FND/FY1/003} HOSPITAL (RF4DG) LDN/RF4DG/FND/FY1/018} HOSPITAL (RF4DG) LDN/RF4DG/FND/FY1/006} NHS FOUNDATION TRUST HOSPITAL -

Capital Hospitals and Barts and the Royal London
Capital Hospitals and Barts and the Royal London Transforming London’s historic hospitals Capital Hospitals and Barts and the Royal London | Transforming London’s historic hospitals Overview Capital Hospitals is a Skanska-led availability Equipment. The 42-year concession with payment-based design, build, finance and Barts and the London NHS* Trust (which was operate (DBFO) Public Private Partnership enlarged and renamed Barts Health NHS Trust (PPP) designed to redevelop two historic in 2012) is the UK’s largest ever healthcare PPP. hospitals in central London. The construction project included two components: Construction on these busy sites was extremely complex, not least because both hospitals had • Demolishing 13 buildings at the Royal to remain fully operational at all times, and was London and creating a new 17-story completed in several phases. building, making it Europe’s largest hospital at the time.. At the Royal London, phase one covered the • Transforming the 900-year-old St demolition of the buildings and the delivery Bartholomew’s into a state-of-the-art cancer of 148,500m2 of new build in three new and cardiac center, demolishing a 1930s towers and was completed in 2011; phase two building and using the site for new build. renovated 15,600m2 and was completed in 2014. Capital Hospitals is also responsible for delivering a very wide range of services At Barts, two phases of work delivered through five further sub-contracts covering: 54,500m2 of new and 13,000m2 of renovated Hard Facilities Management, Soft Facilities space and were completed in March 2010 and Management,, Sterile Services, Managed Equipment, and Radiotherapy Managed *National Health Service Skanska ID Barts and The London Hospitals white paper 2 Capital Hospitals and Barts and the Royal London | Transforming London’s historic hospitals September 2014; a final phase of demolition, The $1.96bn project funding was made up structural work and changes is due for of $1.32bn of Index Linked Bonds issued by completion in February 2016. -

National Cardiac Arrest Audit Participating Hospitals
Updated June 2018 National Cardiac Arrest Audit Participating Hospitals The total number of hospitals signed up to participate in NCAA is 197. England Birmingham and Black Country Non-participant New Cross Hospital The Royal Wolverhampton Hospitals NHS Trust Queen Elizabeth Hospital, Birmingham University Hospital Birmingham NHS Foundation Trust Participant Alexandra Hospital Worcestershire Acute Hospitals NHS Trust Birmingham Heartlands Hospital Heart of England NHS Foundation Trust City Hospital Sandwell and West Birmingham Hospitals NHS Trust Good Hope Hospital Heart of England NHS Foundation Trust Hereford County Hospital Wye Valley NHS Trust Manor Hospital Walsall Healthcare NHS Trust Russells Hall Hospital The Dudley Group of Hospitals NHS Trust Sandwell General Hospital Sandwell and West Birmingham Hospitals NHS Trust Solihull Hospital Heart of England NHS Foundation Trust Worcestershire Royal Hospital Worcestershire Acute Hospitals NHS Trust Central England Participant George Eliot Hospital George Eliot Hospital NHS Trust Glenfield Hospital University Hospitals of Leicester NHS Trust Kettering General Hospital Kettering General Hospital NHS Foundation Trust Leicester General Hospital University Hospitals of Leicester NHS Trust Leicester Royal Infirmary University Hospitals of Leicester NHS Trust Northampton General Hospital Northampton General Hospital NHS Trust Hospital of St Cross, Rugby University Hospitals Coventry and Warwickshire NHS Trust University Hospital Coventry University Hospitals Coventry and Warwickshire NHS Trust -
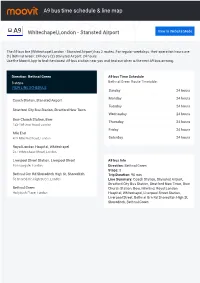
A9 Bus Time Schedule & Line Route
A9 bus time schedule & line map A9 Whitechapel,London - Stansted Airport View In Website Mode The A9 bus line (Whitechapel,London - Stansted Airport) has 2 routes. For regular weekdays, their operation hours are: (1) Bethnal Green: 24 hours (2) Stansted Airport: 24 hours Use the Moovit App to ƒnd the closest A9 bus station near you and ƒnd out when is the next A9 bus arriving. Direction: Bethnal Green A9 bus Time Schedule 8 stops Bethnal Green Route Timetable: VIEW LINE SCHEDULE Sunday 24 hours Monday 24 hours Coach Station, Stansted Airport Tuesday 24 hours Stratford City Bus Station, Stratford New Town Wednesday 24 hours Bow Church Station, Bow Thursday 24 hours 153-159 Bow Road, London Friday 24 hours Mile End 415 Mile End Road, London Saturday 24 hours Royal London Hospital, Whitechapel 257 Whitechapel Road, London Liverpool Street Station, Liverpool Street A9 bus Info Bishopsgate, London Direction: Bethnal Green Stops: 8 Bethnal Grn Rd Shoreditch High St, Shoreditch Trip Duration: 90 min 56 Shoreditch High Street, London Line Summary: Coach Station, Stansted Airport, Stratford City Bus Station, Stratford New Town, Bow Bethnal Green Church Station, Bow, Mile End, Royal London Hollybush Place, London Hospital, Whitechapel, Liverpool Street Station, Liverpool Street, Bethnal Grn Rd Shoreditch High St, Shoreditch, Bethnal Green Direction: Stansted Airport A9 bus Time Schedule 8 stops Stansted Airport Route Timetable: VIEW LINE SCHEDULE Sunday 24 hours Monday 24 hours Royal London Hospital, Whitechapel 257 Whitechapel Road, London Tuesday -

Name Affiliation Professional Role Faisal Abbasi Queen's Hospital
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Frontline Gastroenterol Name Affiliation Professional role Faisal Abbasi Queen’s Hospital Romford, Gastroenterology registrar London, UK Jane Abbot Newham Hospital, London, UK Gastroenterology registrar Omer Ahmad University College London Gastroenterology registrar Hospital, London, UK Ali Akram Ealing Hospital, London, UK Gastroenterology registrar Maria Aslam University College London Gastroenterology registrar Hospital, London, UK Homira Ayubi Croydon University Hospital, Gastroenterology registrar London, UK Jennifer Clough Guy’s and St Thomas’ Hospitals, Gastroenterology registrar London, UK; King’s College London, UK Robin Dart Newham Hospital, London, UK; Gastroenterology registrar King’s College London, UK and visiting research fellow Angad Dhillon St Mark’s Hospital, London, UK Gastroenterology registrar Jonathan Digby- Chelsea and Westminster Gastroenterology registrar Bell Hospital, London, UK Robert Eckersley King George Hospital, London, Gastroenterology registrar UK Tareq El Royal Free Hospital, London, UK Gastroenterology registrar Menabawey Jemima Finkel University College London Gastroenterology registrar Hospital, London, UK Rishi Fofaria St Mark’s Hospital, London, UK Gastroenterology registrar Radha Gadhok Royal London Hospital, London, Gastroenterology registrar UK Shraddha Gulati Royal Free Hospital, London, UK -

The Expansion and Remodelling of the London Hospital by Rowland Plumbe, 1884–1919
For a special issue of The London Journal: A Review of Metropolitan Society Past and Present, 2019 The expansion and remodelling of the London Hospital by Rowland Plumbe, 1884–1919 Amy Smith Survey of London The Bartlett School of Architecture University College London 22 Gordon Street London WC1H 0QB Email: [email protected] The remodelling and enlargement of the London Hospital between 1884 and 1919 by the architect Rowland Plumbe modernized one of the largest general hospitals in Britain. The work almost entirely concealed the mid eighteenth-century core of the hospital and extended its footprint beyond its immediate grounds, creating a sprawling medical complex on the south side of Whitechapel Road. The transformative effect of Plumbe’s work was centred on procuring sanitary and functional interiors. During an association that spanned thirty-five years, Plumbe designed well-ventilated wards, bright operating theatres, and highly specialized departments. Nurses’ homes were planned to preserve discipline and respectability, forming a cluster of tall dormitory blocks connected by covered bridges. This essay, based on research for the Survey of London, considers the medical expertise, organizational principles and social values that shaped a complicated building programme to upgrade an historic infirmary into a hygienic, well-supervised and efficient hospital complex. 1 The London Hospital secured its status as one of the largest general hospitals in Britain in the second half of the nineteenth century. This took time. The institution traced its origins to 1740, when a group of medical men and merchants established a charitable infirmary for the relief of sailors and labourers in east London. -

The Philpot Street Terrace, Whitechapel
The Philpot Street Terrace, Whitechapel A refurbishment and investment opportunity within moments of Whitechapel Overground, Underground and Crossrail Stations close to the Royal London Hospital, Whitechapel, London E1 Philpot Street Terrace Investment Summary • Located close to Whitechapel Overground, Underground and Crossrail station. • Freehold interest in an attractive terrace of Listed buildings near to The Royal London Hospital bounded by Philpot Street, Newark Street and Heron Tower Tower 42 Ashfield Street, London E1. 30 St Mary Axe • A mix of vacant and tenanted office, residential and Liverpool Street Underground, Overground and Crossrail medical accommodation. • Current rental income of £92,400 per annum approximately. • A number of imminent lease expiries in respect of Spitalfields Shoreditch commercial lettings with assured short hold Aldgate East Brick Lane tenancies on the residential elements. • An attractive terrace of properties adjacent to a major refurbishment and redevelopment site immediately to the south, recently purchased from the same vendor, Barts and the London Charity. • An opportunity for refurbishment of the existing residential units, and scope for conversion of office buildings back to residential use (subject to necessary consents). • Offers sought for the freehold interest held by Barts and the London Charity (BtLC). Whitechapel Underground, Overground and Crossrail O2 Canary Wharf Philpot Street Terrace Whitechapel Overground, Royal London Hospital Underground and Crossrail Philpot Street Terrace Royal London Hospital Philpot Street Terrace 1 Philpot Street Terrace Location The Philpot Street Terrace site is an interesting mixed Shoreditch use opportunity immediately to the south of the Royal London Hospital and within minutes walk of Whitechapel Underground and within minutes walk of Aldgate Underground (District, and Hammersmith Liverpool Street and City), Overground and future Crossrail Stations. -

Limehouse Cut and Its Towpath Walks, to Live – and Can Afford To
L IMEHOUSE CUT E14 F R O M LIMEHOUSE’S INDUSTRIAL HERITAGE… TO TODAY’S LONDON LIFESTYLES Computer generated image created for planning purposes. Subject to change. LIMEHOUSE REBORN hoenix is an exciting collection of Pnew 1, 2 & 3 bedroom apartments by Fairview New Homes, just over a mile from Canary Wharf in the heart of London’s thriving East End. Today, with the importance of Canary Wharf and the booming Docklands economy, Limehouse stands on the brink of a prosperous future. New workshops and studios, together with high quality residential development, are bringing new life and excitement to the neighbourhood. WEST HAM A11 BOW ROAD QUEEN MARY UNIVERSITY OF BROMLEY-BY-BOW LONDON MILE END A 1 2 1 A B 1 1 9 0 A1 L 1 A DEVONS ROAD 1 1 C A K 1 W 2 0 A 5 L 2 L 9 T 12 U N 10 N 11 E 140 140 L B B 2 N O 13 R R T I V H E 5 E R R L A E N A 1 LANGDON PARK 2 A 0 P 5 3 P R 8 1 O A 2 A C CANNING TOWN 1 1 H 0 LIMEHOUSE 1 4 6 1 POPLAR 16 A13 3 LIMEHOUSE LIM EH O E U AST INDIA DOCK ROAD S 3 ALL SAINTS A1203 E 2 L I N K 17 T EAST INDIA U 3 4 N BLACKWALL R I V E N R E T H L POPLAR A M E 7 3 4 S A1261 R T H A 8 1 V E M 6 R I E S CANARY WHARF PIER FERRY 5 TERMINAL 5 10 CANARY 7 CANARY WHARF WHARF 6 6 0 0 CANARY WHARF 2 2 18 1 1 A A 15 2 6 14 NORTH GREENWICH SOUTH QUAY Restaurants Schools Shopping Leisure 1 The Orange Room 1 St Saviour’s Church of England 1 Billingsgate Market 1 Lansbury Amateur Boxing Club 10 Mile End Park Stadium 2 Ariana Restaurant Primary School 2 M&S Canary Wharf 2 Poplar Baths Leisure Centre 11 Revolution Karting Go-Cart Track 3 Rum -

RLH Hospital
VARDEN STREET STREET The Royal London Hospital 4 AVELL C Whitechapel 1 London E1 1BB 5 3 Switchboard: 020 7377 7000 WALDEN STREET VARDEN 2 7 STREET 6 STREET PHILPOT STREET 8 ASHFIELD 9 10 WALDEN walk) STREET TURNER STREET mins 11 BETHNAL15 GREEN 20 12 TO 14 TO SHADWELL (approx 19 13 (Approx 12 mins NEW walk) STREET 18 ARK ROAD STREET AVELL 15 C 21 NEW 22 16 33 34 STREET 23 17 31 STEPNEY 32 WA 1 Main building EAST MOUNT Y 30 29 2 Pathology and Pharmacy 3 56-76 Ashfield Street 28 24 4 John Harrison House 5 School of Nursing and Midwifery TURNER 6 Student's Hostel 25 7 43-55 Ashfield Street 8 Postgraduate Centre 26 9 26 Ashfield Street and James Hora Home 10 59-67 Philpott Street 11 Health and Wellness Centre WHITECHAPEL 12 25-29 Ashfield Street WHITECHAPEL 27 13 Charitable Foundation (Approx 1 mins walk) 14 Medical School ROAD 15 Clinical Sciences Research Centre (A11) 16 Dental Hospital and School ROAD 17 Student Union 18 St Philip's Church and Hospital Museum NEW 19 Fielden House 20 St Philip's House 21 Garden House 22 David Hughes 23 Medical School and Old Library TO ALDGA 24 Outpatients Department (Approx 12 mins walk) 25 Outpatients Annexe TE EAST 26 Ambrose King Centre 27 Walk-in Centre 28 Alex Wing 29 West Wing 30 Front Block 31 East Wing 32 Grocer's Wing 33 Holland Wing 34 Link Block Pedestrian access only No access New Hospital at The Royal London Whitechapel To the Ambrose King Centre WHITECHAPEL ROAD and the Dental Institute EAST MOUNT STREET RAVEN ROW Entrance from A&E Entrance Whitechapel Road 9 1 Ambulance Entrance CAVELL -

Barts Health NHS Trust Case Study
Barts Health NHS Trust Case Study Rationalising three different LIMS into a single WinPath Enterprise solution for Barts Health NHS Trust THE CHALLENGE THE OUTCOMES CUSTOMER PROFILE To enable pathology across four Unified methodologies across all 26 million tests per year hospitals to work together hospitals and laboratories in the collaboratively and provide an network, delivering improved 27 labs over 5 sites enhanced service for clinicians, patient care through better offering patients access to a turnaround times, standard 600 staff centre of excellence reference ranges and optimised One of the largest NHS incorporating all aspects of a workflows, in line with the Carter quality pathology service. Report. Trusts Barts Health NHS Trust has one of the largest pathology units in the country, with over 600 staff performing more than 26 million tests per year – the equivalent of 2.5% of the total NHS pathology workload. Barts Health Pathology service is a comprehensive UKAS accredited pathology provider, occupying a purpose built facility located at the Royal London Hospital. They currently operate from four major hospital sites (Royal London, St Bartholomew’s, Whipps Cross and Newham) and at satellite locations (Mile End hospital). The service has been configured as a hub and spoke model and offers a wide repertoire of specialist services, including the main pathology disciplines, Clinical Transplantation, Molecular Haematology, Retinoblastoma, Flow Cytometry and Genomics. CliniSys’ WinPath LIMS has been an enduring part of the pathology -

List of Neurocentres in the UK
List of Neurocentres in the UK Contents • Neurosurgery: p 1 • Neurology departments: p 5 • Radiosurgery and Radiotherapy: p 8 • Spinal Injuries Units: p 10 Neurosurgery Please see below a list of neurosurgery centres in the UK. Neurosurgery centres undertake neurosurgery, in addition to providing general neurology services. East England Addenbrooke's Hospital Hills Road Cambridge CB2 0QQ Queen's Hospital Rom Valley Way Romford Essex RM7 0AG East Midlands Queen's Medical Centre Derby Road Nottingham NG7 2UH Greater London Charing Cross Hospital Fulham Palace Road London W6 8RF Cromwell Hospital (private) Cromwell Road London SW5 0TU 1 Kings College Hospital Denmark Hill London SE5 9RS The National Hospital for Neurology and Neurosurgery Queen Square London WC1N 3BG The Royal Free Pond Street London NW3 2QG Barts and the London Centre for Neurosciences Royal London Hospital Whitechapel Road London E1 1BB St George's Hospital Blackshaw Road London SW17 0QT The Wellington Hospital (private) Wellington Place London NW8 9LE North East England James Cook University Hospital Marton Road Middlesbrough TS4 3BW Regional Neurosciences Centre, Royal Victoria Infirmary Queen Victoria Road Newcastle upon Tyne NE1 4LP North West England Greater Manchester Neurosciences Centre Salford Royal NHS Foundation Trust Stott Lane Salford M6 8HD 2 Royal Preston Hospital Sharoe Green Lane Fulwood Preston PR2 9HT Chorley and South Ribble Hospital Preston Road Chorley PR7 1PP The Walton Centre for Neurology and Neurosurgery Lower Lane Fazakerley Liverpool L9 7LJ South -

Delivering High-Quality Hospital Health Services for the People of North East London Consultation Document November 2009 – March 2010
Delivering high-quality hospital health services for the people of north east London Consultation document November 2009 – March 2010 Health for north east London Aneurin Bevan House, 81 Commercial Road, London E1 1RD. Transforming hospital services OUR PROPOSALS 29 in north east London 5 Contents 7. Our proposals 29 A clinically led process 6 Proposals for complex care on fewer sites 30 This consultation asks for your views 1. Summary 7 on specific proposals that we Proposals for sugery and care believe could provide better Celebrating success 8 for children 31 healthcare in north east London The reasons for change 8 Our proposal for separating planned The proposals 10 operations from emergency care 32 1 2 Finance 12 Major acute hospitals 32 2. How to make your views known 13 Proposals for improving emergency, critical and maternity delivery care 33 BACKGROUND 14 The vision for King George Hospital 38 8. Finance and value for money 40 3. We have listened to your view 14 9. Glossary 42 3 4 4. We already making improvements 16 We are focusing on preventing ill health YOUR VIEWS 45 – as prevention is better than cure… 16 …and we are investing in better Consultation questionnaire 45 services in the community 16 How to give your comments 45 Hospital care is now better than ever 19 5 6 5. More services need to change 21 OUR VISION 24 6. What would first-class NHS services look like? 24 Care and advice close to home 25 7 8 Provide complex care on fewer sites 25 Separate planned operations from emergency services 25 Emergency and critical care