Fossil Calibrations for the Cockroach Phylogeny (Insecta, Dictyoptera, Blattodea)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Aspects About Supella Longipalpa (Blattaria: Blattellidae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Asian Pac J Trop Biomed 2016; 6(12): 1065–1075 1065 HOSTED BY Contents lists available at ScienceDirect Asian Pacific Journal of Tropical Biomedicine journal homepage: www.elsevier.com/locate/apjtb Review article http://dx.doi.org/10.1016/j.apjtb.2016.08.017 New aspects about Supella longipalpa (Blattaria: Blattellidae) Hassan Nasirian* Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran ARTICLE INFO ABSTRACT Article history: The brown-banded cockroach, Supella longipalpa (Blattaria: Blattellidae) (S. longipalpa), Received 16 Jun 2015 recently has infested the buildings and hospitals in wide areas of Iran, and this review was Received in revised form 3 Jul 2015, prepared to identify current knowledge and knowledge gaps about the brown-banded 2nd revised form 7 Jun, 3rd revised cockroach. Scientific reports and peer-reviewed papers concerning S. longipalpa and form 18 Jul 2016 relevant topics were collected and synthesized with the objective of learning more about Accepted 10 Aug 2016 health-related impacts and possible management of S. longipalpa in Iran. Like the Available online 15 Oct 2016 German cockroach, the brown-banded cockroach is a known vector for food-borne dis- eases and drug resistant bacteria, contaminated by infectious disease agents, involved in human intestinal parasites and is the intermediate host of Trichospirura leptostoma and Keywords: Moniliformis moniliformis. Because its habitat is widespread, distributed throughout Brown-banded cockroach different areas of homes and buildings, it is difficult to control. -

Cómo Citar El Artículo Número Completo Más Información Del
Acta zoológica mexicana ISSN: 0065-1737 ISSN: 2448-8445 Instituto de Ecología A.C. García, Mauricio; Camacho, Jesús; Dorado, Idelma Dos nuevas especies de Termitaradus Myers, 1924 (Hemiptera: Termitaphididae), de Venezuela y observaciones sobre la familia Acta zoológica mexicana, vol. 32, núm. 3, 2016, pp. 348-358 Instituto de Ecología A.C. Disponible en: http://www.redalyc.org/articulo.oa?id=57549165012 Cómo citar el artículo Número completo Sistema de Información Científica Redalyc Más información del artículo Red de Revistas Científicas de América Latina y el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto ISSN 0065-1737 (NUEVA SERIE) 32(3) 2016 DOS NUEVAS ESPECIES DE TERMITARADUS MYERS, 1924 (HEMIPTERA: TERMITAPHIDIDAE), DE VENEZUELA Y OBSERVACIONES SOBRE LA FAMILIA TWO NEW SPECIES OF TERMITARADUS MYERS, 1924 (HEMIPTERA: TERMITAPHIDIDAE) OF VENEZUELA, AND OBSERVATIONS ON THE FAMILY Mauricio GARCÍA,¹ Jesús CAMACHO² e Idelma DORADO² ¹ Centro de Investigaciones Biológicas (CIB), Facultad de Humanidades y Educación, Edificio de Postgrado, Universidad del Zulia, Apdo. 526, A-4001, Venezuela. ² Museo de Artrópodos de la Universidad del Zulia (MALUZ). Departamento Fitosanitario, Facultad de Agronomía, Universidad del Zulia, Apdo. 526, Maracaibo A-4001, Zulia, Venezuela. <[email protected]>; <[email protected]>; <[email protected]> Recibido: 28/03/2016; aceptado: 30/08/2016 Editor asociado responsable: Alfonso Neri García Aldrete García, M., Camacho, J. & Dorado, I. (2016). Dos nuevas especies García, M., Camacho, J. & Dorado, I. (2016). Two new species of de Termitaradus Myers, 1924 (Hemiptera: Termitaphididae), de Termitaradus Myers, 1924 (Hemiptera: Termitaphididae) of Ven- Venezuela y observaciones sobre la familia. -

Cockroach Marion Copeland
Cockroach Marion Copeland Animal series Cockroach Animal Series editor: Jonathan Burt Already published Crow Boria Sax Tortoise Peter Young Ant Charlotte Sleigh Forthcoming Wolf Falcon Garry Marvin Helen Macdonald Bear Parrot Robert E. Bieder Paul Carter Horse Whale Sarah Wintle Joseph Roman Spider Rat Leslie Dick Jonathan Burt Dog Hare Susan McHugh Simon Carnell Snake Bee Drake Stutesman Claire Preston Oyster Rebecca Stott Cockroach Marion Copeland reaktion books Published by reaktion books ltd 79 Farringdon Road London ec1m 3ju, uk www.reaktionbooks.co.uk First published 2003 Copyright © Marion Copeland All rights reserved No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior permission of the publishers. Printed and bound in Hong Kong British Library Cataloguing in Publication Data Copeland, Marion Cockroach. – (Animal) 1. Cockroaches 2. Animals and civilization I. Title 595.7’28 isbn 1 86189 192 x Contents Introduction 7 1 A Living Fossil 15 2 What’s in a Name? 44 3 Fellow Traveller 60 4 In the Mind of Man: Myth, Folklore and the Arts 79 5 Tales from the Underside 107 6 Robo-roach 130 7 The Golden Cockroach 148 Timeline 170 Appendix: ‘La Cucaracha’ 172 References 174 Bibliography 186 Associations 189 Websites 190 Acknowledgements 191 Photo Acknowledgements 193 Index 196 Two types of cockroach, from the first major work of American natural history, published in 1747. Introduction The cockroach could not have scuttled along, almost unchanged, for over three hundred million years – some two hundred and ninety-nine million before man evolved – unless it was doing something right. -

UFRJ a Paleoentomofauna Brasileira
Anuário do Instituto de Geociências - UFRJ www.anuario.igeo.ufrj.br A Paleoentomofauna Brasileira: Cenário Atual The Brazilian Fossil Insects: Current Scenario Dionizio Angelo de Moura-Júnior; Sandro Marcelo Scheler & Antonio Carlos Sequeira Fernandes Universidade Federal do Rio de Janeiro, Programa de Pós-Graduação em Geociências: Patrimônio Geopaleontológico, Museu Nacional, Quinta da Boa Vista s/nº, São Cristóvão, 20940-040. Rio de Janeiro, RJ, Brasil. E-mails: [email protected]; [email protected]; [email protected] Recebido em: 24/01/2018 Aprovado em: 08/03/2018 DOI: http://dx.doi.org/10.11137/2018_1_142_166 Resumo O presente trabalho fornece um panorama geral sobre o conhecimento da paleoentomologia brasileira até o presente, abordando insetos do Paleozoico, Mesozoico e Cenozoico, incluindo a atualização das espécies publicadas até o momento após a última grande revisão bibliográica, mencionando ainda as unidades geológicas em que ocorrem e os trabalhos relacionados. Palavras-chave: Paleoentomologia; insetos fósseis; Brasil Abstract This paper provides an overview of the Brazilian palaeoentomology, about insects Paleozoic, Mesozoic and Cenozoic, including the review of the published species at the present. It was analiyzed the geological units of occurrence and the related literature. Keywords: Palaeoentomology; fossil insects; Brazil Anuário do Instituto de Geociências - UFRJ 142 ISSN 0101-9759 e-ISSN 1982-3908 - Vol. 41 - 1 / 2018 p. 142-166 A Paleoentomofauna Brasileira: Cenário Atual Dionizio Angelo de Moura-Júnior; Sandro Marcelo Schefler & Antonio Carlos Sequeira Fernandes 1 Introdução Devoniano Superior (Engel & Grimaldi, 2004). Os insetos são um dos primeiros organismos Algumas ordens como Blattodea, Hemiptera, Odonata, Ephemeroptera e Psocopera surgiram a colonizar os ambientes terrestres e aquáticos no Carbonífero com ocorrências até o recente, continentais (Engel & Grimaldi, 2004). -

Fossil Insects.*
ANNALS OF The Entomological Society of America Downloaded from https://academic.oup.com/aesa/article/10/1/1/8284 by guest on 25 September 2021 Volume X MARCH, 19 17 Number 1 FOSSIL INSECTS.* By T. D. A. COCKERELL. In these serious days, it seems just a little grotesque that I should cross half a continent to address you on a subject so remote from the current of human life as fossil insects. The limitations of our society do indeed forbid such topics as the causes of the war or the evil effects of intercollegiate athletics; but I might have chosen to discuss lice or mosquitoes—any of those insects whose activities have before now decided the fate of nations. My excuse for avoiding these more lively topics only aggravates the offense, for it is the fact that I have never given them adequate attention, but have in the past ten years occupied myself with matters having for the most part no obvious economic application. There is, however, another point of view. Many years ago I had the good fortune to meet the eminent ornithologist, Elliott Coues, at Santa Fe. We spent a considerable part of the night discussing a variety of subjects, from spiritualism to rattlesnakes, and when we parted he made a remark which those who knew him will recognize as characteristic. He said, "Cockerell, I really believe that if it had not been for science, you would have been a dangerous crank!" Surely experience and history alike confirm the essential sagacity of the observa tion, as applied not merely to your lecturer, but to mankind in general. -

Evaluation of the Chemical Defense Fluids of Macrotermes Carbonarius
www.nature.com/scientificreports OPEN Evaluation of the chemical defense fuids of Macrotermes carbonarius and Globitermes sulphureus as possible household repellents and insecticides S. Appalasamy1,2*, M. H. Alia Diyana2, N. Arumugam2 & J. G. Boon3 The use of chemical insecticides has had many adverse efects. This study reports a novel perspective on the application of insect-based compounds to repel and eradicate other insects in a controlled environment. In this work, defense fuid was shown to be a repellent and insecticide against termites and cockroaches and was analyzed using gas chromatography-mass spectrometry (GC– MS). Globitermes sulphureus extract at 20 mg/ml showed the highest repellency for seven days against Macrotermes gilvus and for thirty days against Periplaneta americana. In terms of toxicity, G. sulphureus extract had a low LC50 compared to M. carbonarius extract against M. gilvus. Gas chromatography–mass spectrometry analysis of the M. carbonarius extract indicated the presence of six insecticidal and two repellent compounds in the extract, whereas the G. sulphureus extract contained fve insecticidal and three repellent compounds. The most obvious fnding was that G. sulphureus defense fuid had higher potential as a natural repellent and termiticide than the M. carbonarius extract. Both defense fuids can play a role as alternatives in the search for new, sustainable, natural repellents and termiticides. Our results demonstrate the potential use of termite defense fuid for pest management, providing repellent and insecticidal activities comparable to those of other green repellent and termiticidal commercial products. A termite infestation could be silent, but termites are known as destructive urban pests that cause structural damage by infesting wooden and timber structures, leading to economic loss. -

Bulletin Number / Numéro 2 Entomological Society of Canada Société D’Entomologie Du Canada June / Juin 2008
Volume 40 Bulletin Number / numéro 2 Entomological Society of Canada Société d’entomologie du Canada June / juin 2008 Published quarterly by the Entomological Society of Canada Publication trimestrielle par la Société d’entomologie du Canada ............................................................... .................................................................................................................................................................................................................................................................................................................................. .......................................................................... ........................................................................................................................................................................ ....................... ................................................................................. ................................................. List of contents / Table des matières Volume 40 (2), June / june 2008 Up front / Avant-propos ................................................................................................................49 Moth balls / Boules à mites .............................................................................................................51 Meeting announcements / Réunions futures ..................................................................................52 Dear Buggy / Cher Bibitte ..............................................................................................................53 -
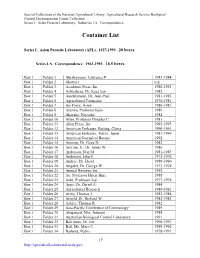
Container List
Special Collections of the National Agricultural Library: Agricultural Research Service Biological Control Documentation Center Collection Series I. Asian Parasite Laboratory. Subseries I.A. Correspondence. Container List Series I. Asian Parasite Laboratory (APL). 1927-1993. 20 boxes. Series I.A. Correspondence. 1963-1993. 16.5 boxes. Box 1 Folder 1 Abrahamson, Lawrence P. 1983-1984 Box 1 Folder 2 Abstract n.d. Box 1 Folder 3 Academic Press, Inc. 1986-1993 Box 1 Folder 4 Achterberg, Dr. Kees van 1982 Box 1 Folder 5 Aeschlimann, Dr. Jean-Paul 1981-1985 Box 1 Folder 6 Agricultural Counselor 1976-1981 Box 1 Folder 7 Air Force, Army 1980-1981 Box 1 Folder 8 Aizawa, Professor Keio 1986 Box 1 Folder 9 Akasaka, Naoyuki 1982 Box 1 Folder 10 Allen, Professor Douglas C. 1981 Box 1 Folder 11 Allen Press, Inc. 1992-1993 Box 1 Folder 12 American Embassy, Beijing, China 1990-1991 Box 1 Folder 13 American Embassy, Tokyo, Japan 1981-1990 Box 1 Folder 14 American Journal of Botany 1992 Box 1 Folder 15 Amman, Dr. Gene D. 1982 Box 1 Folder 16 Amrine, Jr., Dr. James W. 1986 Box 1 Folder 17 Anderson, Don M. 1981-1983 Box 1 Folder 18 Anderson, John F. 1975-1976 Box 1 Folder 19 Andres, Dr. Lloyd 1989-1990 Box 1 Folder 20 Angalet, Dr. George W. 1973-1978 Box 1 Folder 21 Annual Reviews Inc. 1992 Box 1 Folder 22 Ao, Professor Hsien-Bine 1988 Box 1 Folder 23 Aoki, Professor Joji 1977-1978 Box 1 Folder 24 Apps, Dr. Darrel A. 1984 Box 1 Folder 25 Agricultural Research 1980-1981 Box 1 Folder 26 Army, Thomas J. -

Movement of Plastic-Baled Garbage and Regulated (Domestic) Garbage from Hawaii to Landfills in Oregon, Idaho, and Washington
Movement of Plastic-baled Garbage and Regulated (Domestic) Garbage from Hawaii to Landfills in Oregon, Idaho, and Washington. Final Biological Assessment, February 2008 Table of Contents I. Introduction and Background on Proposed Action 3 II. Listed Species and Program Assessments 28 Appendix A. Compliance Agreements 85 Appendix B. Marine Mammal Protection Act 150 Appendix C. Risk of Introduction of Pests to the Continental United States via Municipal Solid Waste from Hawaii. 159 Appendix D. Risk of Introduction of Pests to Washington State via Municipal Solid Waste from Hawaii 205 Appendix E. Risk of Introduction of Pests to Oregon via Municipal Solid Waste from Hawaii. 214 Appendix F. Risk of Introduction of Pests to Idaho via Municipal Solid Waste from Hawaii. 233 2 I. Introduction and Background on Proposed Action This biological assessment (BA) has been prepared by the United States Department of Agriculture (USDA), Animal and Plant Health Inspection Service (APHIS) to evaluate the potential effects on federally-listed threatened and endangered species and designated critical habitat from the movement of baled garbage and regulated (domestic) garbage (GRG) from the State of Hawaii for disposal at landfills in Oregon, Idaho, and Washington. Specifically, garbage is defined as urban (commercial and residential) solid waste from municipalities in Hawaii, excluding incinerator ash and collections of agricultural waste and yard waste. Regulated (domestic) garbage refers to articles generated in Hawaii that are restricted from movement to the continental United States under various quarantine regulations established to prevent the spread of plant pests (including insects, disease, and weeds) into areas where the pests are not prevalent. -

Long Rdna Amplicon Sequencing of Insect-Infecting Nephridiophagids
www.nature.com/scientificreports OPEN Long rDNA amplicon sequencing of insect‑infecting nephridiophagids reveals their afliation to the Chytridiomycota and a potential to switch between hosts Jürgen F. H. Strassert 1*, Christian Wurzbacher 2, Vincent Hervé 3, Taraha Antany1, Andreas Brune 3 & Renate Radek 1* Nephridiophagids are unicellular eukaryotes that parasitize the Malpighian tubules of numerous insects. Their life cycle comprises multinucleate vegetative plasmodia that divide into oligonucleate and uninucleate cells, and sporogonial plasmodia that form uninucleate spores. Nephridiophagids are poor in morphological characteristics, and although they have been tentatively identifed as early‑branching fungi based on the SSU rRNA gene sequences of three species, their exact position within the fungal tree of live remained unclear. In this study, we describe two new species of nephridiophagids (Nephridiophaga postici and Nephridiophaga javanicae) from cockroaches. Using long‑read sequencing of the nearly complete rDNA operon of numerous further species obtained from cockroaches and earwigs to improve the resolution of the phylogenetic analysis, we found a robust afliation of nephridiophagids with the Chytridiomycota—a group of zoosporic fungi that comprises parasites of diverse host taxa, such as microphytes, plants, and amphibians. The presence of the same nephridiophagid species in two only distantly related cockroaches indicates that their host specifcity is not as strict as generally assumed. Insects are the most diverse group of all animals. So far, about one million species have been described and recent estimates for extant species range from 2.6 to 7.8 million1,2. Tey are globally distributed and impact human life at numerous levels. In agriculture, for instance, insects play a major role as both pollinators (e.g., honey bees) and pests that feed on crops (e.g., grasshoppers). -

V·M·I University Microfilms International a Beil & Howell Information Company 300 North Zeeb Road
INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adverselyaffect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. V·M·I University Microfilms International A Beil & Howell Information Company 300 North Zeeb Road. Ann Arbor. M148106-1346 USA 313'761-4700 800,521-0600 Order Number 9215026 Energy allocation and reproductive effort in four cockroach species with differing modes of reproduction Koebele, Bruce Peter, Ph.D. University of Hawaii, 1991 Copyright @1991 by Koebele, Bruce Peter. -

The Dusky Cockroach in the Canadian Maritimes: Establishment, Persistence, and Ecology Jeff C
J. Acad. Entomol. Soc. 13: 21-27 (2017) The Dusky Cockroach in the Canadian Maritimes: establishment, persistence, and ecology Jeff C. Clements, David B. McCorquodale, Denis A. Doucet, Jeffrey B. Ogden ABSTRACT The Dusky Cockroach, Ectobius lapponicus (Linnaeus, 1758) (Blattodea: Blatellidae), a European native, is an introduced species in North America that was first discovered in New Hampshire in 1984. In Canada, this species was first found in Prince Edward Island in 1991 and has recently been recorded in all three Maritime Provinces. Using ad libitum reports of Ectobius lapponicus sightings with confirmed species identification, we provide an update for an earlier postulation of the establishment and persistence of this non-native cockroach in the Canadian Maritimes, highlighting spatial and temporal trends in Ectobius lapponicus records. While a 13-year gap exists after its original Canadian record in 1991, Ectobius lapponicus has been observed in the Maritimes almost annually since 2004. To date, a total of 119 Ectobius lapponicus individuals have been reported in the Canadian Maritimes: 45 from New Brunswick, 38 from Nova Scotia, and 36 from Prince Edward Island. Seventy-eight percent of individuals are reported from tourist destinations (parks and campgrounds). The vast majority of individuals have been observed outdoors in disturbed habitats near forest edges, although some indoor records exist. Records suggest that this species is active from June–September, which is in accordance with typical periods of activity in Europe. This species also appears well established in Ontario. Widespread confirmation of this species throughout the state of Maine supports the northward spread of this species from New Hampshire into the Canadian Maritimes, likely driven by human-assisted dispersal.