Induced Rituximab- but Not GA101 KIR/HLA Interactions Negatively Affect
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prescribing Information: Gazyvaro® (Obinutuzumab)
Prescribing information: Gazyvaro®▼ (obinutuzumab) PRESCRIBING INFORMATION Gazyvaro maintenance as a single agent, 1000 mg 70 mL/min are more at risk of IRRs, including severe Gazyvaro® (obinutuzumab) 1000 mg once every 2 months for 2 years or until disease IRRs. Do not administer further infusions if patient concentrate for solution for infusion progression (whichever occurs first). Administration: experiences acute life-threatening respiratory Refer to Gazyvaro Summary of Product Monitor closely for infusion related reactions (IRRs). symptoms, a Grade 4 (life threatening) IRR or, a Characteristics (SmPC) for full prescribing CLL: Cycle 1: Day 1(100 mg): Administer at 25 mg/hr second occurrence of a Grade 3 (prolonged/recurrent) information. over 4 hours. Do not increase the infusion rate. Day 2 IRR (after resuming the first infusion or during a (or Day 1 continued) (900 mg): If no IRR occurred subsequent infusion). Carefully monitor patients who Indications: Previously-untreated chronic lymphocytic during prior infusion administer at 50 mg/hr. Infusion have pre-existing cardiac or pulmonary conditions leukaemia (CLL), in combination with chlorambucil, in rate can be escalated in 50 mg/hr increments every 30 throughout the infusion and post-infusion period. For patients with co-morbidities making them unsuitable for minutes to 400 mg/hr. – All subsequent infusions: If no patients at acute risk of hypertensive crisis evaluate full-dose fludarabine-based therapy. Follicular IRR occurred during prior infusion when final rate was the benefit and risks of withholding anti-hypertensive lymphoma (FL), in combination with bendamustine 100 mg/hr or faster, start at 100 mg/hr and increase by medicine. -

Assessment of Overall Survival, Quality of Life, And
Supplementary Online Content Salas-Vega S, Iliopoulos O, Mossialos E. Assesment of overall survival, quality of life, and safety benefits associated with new cancer medicines [published online December 29, 2016]. JAMA Oncol. doi:10.1001/jamaoncol.2016.4166 eTable 1. Therapeutic profile of all cancer medicines approved by the FDA between 2003- 2013 eTable 2. Regulatory evidence in support of classification of drug clinical benefits eFigure 1. Number of cancer drugs that were evaluated by all three HTA agencies, sorted by magnitude of clinical benefits eTable 3. Interagency agreement–Krippendorff’s alpha coefficients eMethods This supplementary material has been provided by the authors to give readers additional information about their work. Online-Only Supplement © 2016 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/25/2021 Clinical value of cancer medicines Contents eExhibits ......................................................................................................................................................... 3 eTable 1. Therapeutic profile of all cancer medicines approved by the FDA between 2003- 2013 (Summary of eTable 2) ................................................................................................................... 3 eTable 2. Regulatory evidence in support of classification of drug clinical benefits ....................... 6 eFigure 1. Number of cancer drugs that were evaluated by all three HTA agencies, sorted by magnitude of clinical benefits -

Expression Tissue
The Journal of Immunology Killer Cell Ig-Like Receptor and Leukocyte Ig-Like Receptor Transgenic Mice Exhibit Tissue- and Cell-Specific Transgene Expression1 Danny Belkin,* Michaela Torkar,* Chiwen Chang,* Roland Barten,* Mauro Tolaini,† Anja Haude,* Rachel Allen,* Michael J. Wilson,* Dimitris Kioussis,† and John Trowsdale2* To generate an experimental model for exploring the function, expression pattern, and developmental regulation of human Ig-like activating and inhibitory receptors, we have generated transgenic mice using two human genomic clones: 52N12 (a 150-Kb clone encompassing the leukocyte Ig-like receptor (LILR)B1 (ILT2), LILRB4 (ILT3), and LILRA1 (LIR6) genes) and 1060P11 (a 160-Kb clone that contains ten killer cell Ig-like receptor (KIR) genes). Both the KIR and LILR families are encoded within the leukocyte receptor complex, and are involved in immune modulation. We have also produced a novel mAb to LILRA1 to facilitate expression studies. The LILR transgenes were expressed in a similar, but not identical, pattern to that observed in humans: LILRB1 was expressed in B cells, most NK cells, and a small number of T cells; LILRB4 was expressed in a B cell subset; and LILRA1 was found on a ring of cells surrounding B cell areas on spleen sections, consistent with other data showing monocyte/macrophage expression. KIR transgenic mice showed KIR2DL2 expression on a subset of NK cells and T cells, similar to the pattern seen in humans, and expression of KIR2DL4, KIR3DS1, and KIR2DL5 by splenic NK cells. These observations indicate that linked regulatory elements within the genomic clones are sufficient to allow appropriate expression of KIRs in mice, and illustrate that the presence of the natural ligands for these receptors, in the form of human MHC class I proteins, is not necessary for the expression of the KIRs observed in these mice. -
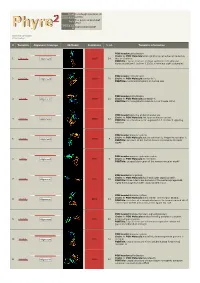
Phyre 2 Results for A1YIY0
Email [email protected] Description A1YIY0 Tue Jul 30 13:19:15 BST Date 2013 Unique Job 1035bc4b501530df ID Detailed template information # Template Alignment Coverage 3D Model Confidence % i.d. Template Information PDB header:cell adhesion Chain: A: PDB Molecule:down syndrome cell adhesion molecule 1 c3dmkA_ Alignment 100.0 14 (dscam) isoform PDBTitle: crystal structure of down syndrome cell adhesion molecule (dscam)2 isoform 1.30.30, n-terminal eight ig domains PDB header:cell adhesion 2 c2om5A_ Alignment 100.0 20 Chain: A: PDB Molecule:contactin 2; PDBTitle: n-terminal fragment of human tax1 PDB header:cell adhesion 3 c3jxaA_ Alignment 100.0 21 Chain: A: PDB Molecule:contactin 4; PDBTitle: immunoglobulin domains 1-4 of mouse cntn4 PDB header:signaling protein/transferase Chain: A: PDB Molecule:tek tyrosine kinase variant; 4 c4k0vA_ 100.0 12 Alignment PDBTitle: structural basis for angiopoietin-1 mediated signaling initiation PDB header:immune system Chain: A: PDB Molecule:natural cytotoxicity triggering receptor 1; 5 c1p6fA_ 100.0 9 Alignment PDBTitle: structure of the human natural cytotoxicity receptor nkp46 PDB header:immune system/receptor 6 c1ollA_ Alignment 99.9 9 Chain: A: PDB Molecule:nk receptor; PDBTitle: extracellular region of the human receptor nkp46 PDB header:viral protein Chain: A: PDB Molecule:hoc head outer capsid protein; 7 c3shsA_ 99.9 18 Alignment PDBTitle: three n-terminal domains of the bacteriophage rb49 highly immunogenic2 outer capsid protein (hoc) PDB header:immune system Chain: E: PDB Molecule:natural -

CD226 T Cells Expressing the Receptors TIGIT and Divergent Phenotypes of Human Regulatory
The Journal of Immunology Divergent Phenotypes of Human Regulatory T Cells Expressing the Receptors TIGIT and CD226 Christopher A. Fuhrman,*,1 Wen-I Yeh,*,1 Howard R. Seay,* Priya Saikumar Lakshmi,* Gaurav Chopra,† Lin Zhang,* Daniel J. Perry,* Stephanie A. McClymont,† Mahesh Yadav,† Maria-Cecilia Lopez,‡ Henry V. Baker,‡ Ying Zhang,x Yizheng Li,{ Maryann Whitley,{ David von Schack,x Mark A. Atkinson,* Jeffrey A. Bluestone,‡ and Todd M. Brusko* Regulatory T cells (Tregs) play a central role in counteracting inflammation and autoimmunity. A more complete understanding of cellular heterogeneity and the potential for lineage plasticity in human Treg subsets may identify markers of disease pathogenesis and facilitate the development of optimized cellular therapeutics. To better elucidate human Treg subsets, we conducted direct transcriptional profiling of CD4+FOXP3+Helios+ thymic-derived Tregs and CD4+FOXP3+Helios2 T cells, followed by comparison with CD4+FOXP32Helios2 T conventional cells. These analyses revealed that the coinhibitory receptor T cell Ig and ITIM domain (TIGIT) was highly expressed on thymic-derived Tregs. TIGIT and the costimulatory factor CD226 bind the common ligand CD155. Thus, we analyzed the cellular distribution and suppressive activity of isolated subsets of CD4+CD25+CD127lo/2 T cells expressing CD226 and/or TIGIT. We observed TIGIT is highly expressed and upregulated on Tregs after activation and in vitro expansion, and is associated with lineage stability and suppressive capacity. Conversely, the CD226+TIGIT2 population was associated with reduced Treg purity and suppressive capacity after expansion, along with a marked increase in IL-10 and effector cytokine production. These studies provide additional markers to delineate functionally distinct Treg subsets that may help direct cellular therapies and provide important phenotypic markers for assessing the role of Tregs in health and disease. -

Eosinophil Cytolysis and Release of Cell-Free Granules
CORRESPONDENCE LINK TO ORIGINAL ARTICLE LINK TO INITIAL CORRESPONDENCE to determine what conditions in vivo favour piecemeal degranulation and what condi- Eosinophil cytolysis and release of tions promote cytolysis (with or without ‘net’ formation) and release of intact granules. cell-free granules There are several strong recent reviews cover- ing mechanisms of degranulation, including 10 Helene F. Rosenberg and Paul S. Foster those written by Neves and Weller , and Lacy and Moqbel11, for those seeking greater insight into this important and evolving field. We are grateful for an excellent oppor- The results of eosinophil cytolysis — Helene F. Rosenberg is in the Inflammation tunity to expand on our recent Review specifically, the release of intact granules — Immunobiology Section, National Institute of Allergy (Eosinophils: changing perspectives in are not new findings. There have been many and Infectious Diseases, National Institutes of Health, health and disease. Nature Rev. Immunol. reports of free granules in tissues found in Bethesda, Maryland 20892, USA. 13, 9–22 (2013))1 that has been provided by conjunction with eosinophil-associated dis- Paul S. Foster is at the Priority Research Center the correspondence from Carl Persson and eases, including allergic rhinitis, bronchial for Asthma and Respiratory Diseases, Hunter Medical Research Institute and School of Lena Uller (Primary lysis of eosinophils asthma, atopic dermatitis, urticaria and Biomedical Sciences and Pharmacy, Faculty of Health, 7 as a major mode of activation of eosino- eosinophilic esophagitis . However, it was University of Newcastle, Newcastle, New South Wales, phils in human diseased tissues. Nature not clear what role these granules had, if any, 2300, Australia. -

Associations Between FCGR3A Polymorphisms and Susceptibility to Rheumatoid Arthritis: a Metaanalysis YOUNG HO LEE, JONG DAE JI, and GWAN GYU SONG
Associations Between FCGR3A Polymorphisms and Susceptibility to Rheumatoid Arthritis: A Metaanalysis YOUNG HO LEE, JONG DAE JI, and GWAN GYU SONG ABSTRACT. Objective. To investigate whether the Fcγ receptor (FCGR) polymorphism confers susceptibility to rheumatoid arthritis (RA). Methods. We conducted metaanalyses on the associations between FCGR polymorphisms and RA susceptibility as determined using (1) allele contrast, (2) recessive models, (3) dominant models, and (4) contrast of homozygotes, using fixed or random effects models. Results. A total of 10 separate comparisons were considered, which comprised 6 European and 4 Asian population samples. Metaanalysis of FCGR3A polymorphism revealed a significant associa- tion between the VV genotype and the risk of developing RA relative to the VF+FF genotype (OR 1.256, 95% CI 1.045–1.510, p = 0.015), with no evidence of between-study heterogeneity (p = 0.167). In subjects of European descent, a stronger association was observed between the VV geno- type and RA than for the FF genotype (OR 1.374, 95% CI 1.101–1.714, p = 0.005). In Asians, no such association was found. Metaanalysis of the VV vs FF genotype revealed a significantly increased OR in Europeans (OR 1.399, 95% CI 1.107–1.769, p = 0.005), but not in Asians. No asso- ciation was found between RA and the FCGR2A and FCGR3B polymorphisms in all subjects and in European and Asian populations, except for the NA22 vs NA11 of FCGR3B in Europeans. Conclusion. No relation was found between the FCGR2A polymorphism and susceptibility to RA in Europeans or Asians. The FCGR3A polymorphism was found to be associated with RA in Europeans but not in Asians. -

Single-Cell RNA Sequencing Demonstrates the Molecular and Cellular Reprogramming of Metastatic Lung Adenocarcinoma
ARTICLE https://doi.org/10.1038/s41467-020-16164-1 OPEN Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma Nayoung Kim 1,2,3,13, Hong Kwan Kim4,13, Kyungjong Lee 5,13, Yourae Hong 1,6, Jong Ho Cho4, Jung Won Choi7, Jung-Il Lee7, Yeon-Lim Suh8,BoMiKu9, Hye Hyeon Eum 1,2,3, Soyean Choi 1, Yoon-La Choi6,10,11, Je-Gun Joung1, Woong-Yang Park 1,2,6, Hyun Ae Jung12, Jong-Mu Sun12, Se-Hoon Lee12, ✉ ✉ Jin Seok Ahn12, Keunchil Park12, Myung-Ju Ahn 12 & Hae-Ock Lee 1,2,3,6 1234567890():,; Advanced metastatic cancer poses utmost clinical challenges and may present molecular and cellular features distinct from an early-stage cancer. Herein, we present single-cell tran- scriptome profiling of metastatic lung adenocarcinoma, the most prevalent histological lung cancer type diagnosed at stage IV in over 40% of all cases. From 208,506 cells populating the normal tissues or early to metastatic stage cancer in 44 patients, we identify a cancer cell subtype deviating from the normal differentiation trajectory and dominating the metastatic stage. In all stages, the stromal and immune cell dynamics reveal ontological and functional changes that create a pro-tumoral and immunosuppressive microenvironment. Normal resident myeloid cell populations are gradually replaced with monocyte-derived macrophages and dendritic cells, along with T-cell exhaustion. This extensive single-cell analysis enhances our understanding of molecular and cellular dynamics in metastatic lung cancer and reveals potential diagnostic and therapeutic targets in cancer-microenvironment interactions. 1 Samsung Genome Institute, Samsung Medical Center, Seoul 06351, Korea. -

Associations Between FCGR3A Polymorphisms and Susceptibility to Rheumatoid Arthritis: a Metaanalysis YOUNG HO LEE, JONG DAE JI, and GWAN GYU SONG
Associations Between FCGR3A Polymorphisms and Susceptibility to Rheumatoid Arthritis: A Metaanalysis YOUNG HO LEE, JONG DAE JI, and GWAN GYU SONG ABSTRACT. Objective. To investigate whether the Fcγ receptor (FCGR) polymorphism confers susceptibility to rheumatoid arthritis (RA). Methods. We conducted metaanalyses on the associations between FCGR polymorphisms and RA susceptibility as determined using (1) allele contrast, (2) recessive models, (3) dominant models, and (4) contrast of homozygotes, using fixed or random effects models. Results. A total of 10 separate comparisons were considered, which comprised 6 European and 4 Asian population samples. Metaanalysis of FCGR3A polymorphism revealed a significant associa- tion between the VV genotype and the risk of developing RA relative to the VF+FF genotype (OR 1.256, 95% CI 1.045–1.510, p = 0.015), with no evidence of between-study heterogeneity (p = 0.167). In subjects of European descent, a stronger association was observed between the VV geno- type and RA than for the FF genotype (OR 1.374, 95% CI 1.101–1.714, p = 0.005). In Asians, no such association was found. Metaanalysis of the VV vs FF genotype revealed a significantly increased OR in Europeans (OR 1.399, 95% CI 1.107–1.769, p = 0.005), but not in Asians. No asso- ciation was found between RA and the FCGR2A and FCGR3B polymorphisms in all subjects and in European and Asian populations, except for the NA22 vs NA11 of FCGR3B in Europeans. Conclusion. No relation was found between the FCGR2A polymorphism and susceptibility to RA in Europeans or Asians. The FCGR3A polymorphism was found to be associated with RA in Europeans but not in Asians. -

Single-Cell RNA Sequencing of Tocilizumab-Treated Peripheral Blood Mononuclear Cells As an in Vitro Model of Inflammation
UCSF UC San Francisco Previously Published Works Title Single-Cell RNA Sequencing of Tocilizumab-Treated Peripheral Blood Mononuclear Cells as an in vitro Model of Inflammation. Permalink https://escholarship.org/uc/item/8q84n3jb Authors Zarinsefat, Arya Hartoularos, George Rychkov, Dmitry et al. Publication Date 2020 DOI 10.3389/fgene.2020.610682 Peer reviewed eScholarship.org Powered by the California Digital Library University of California fgene-11-610682 December 19, 2020 Time: 19:44 # 1 BRIEF RESEARCH REPORT published: 05 January 2021 doi: 10.3389/fgene.2020.610682 Single-Cell RNA Sequencing of Tocilizumab-Treated Peripheral Blood Mononuclear Cells as an in vitro Model of Inflammation Arya Zarinsefat1*, George Hartoularos2, Dmitry Rychkov1, Priyanka Rashmi1, Sindhu Chandran3, Flavio Vincenti1, Chun J. Yee2 and Minnie M. Sarwal1 1 Department of Surgery, University of California, San Francisco, San Francisco, CA, United States, 2 Department of Bioengineering and Therapeutic Sciences, University of California, San Francisco, San Francisco, CA, United States, 3 Department of Medicine, University of California, San Francisco, San Francisco, CA, United States COVID-19 has posed a significant threat to global health. Early data has revealed that IL-6, a key regulatory cytokine, plays an important role in the cytokine storm of COVID-19. Multiple trials are therefore looking at the effects of Tocilizumab, an IL-6 receptor antibody that inhibits IL-6 activity, on treatment of COVID-19, with promising findings. As part of a clinical trial looking at the effects of Tocilizumab treatment on Edited by: kidney transplant recipients with subclinical rejection, we performed single-cell RNA Gabriele Bucci, sequencing of comparing stimulated PBMCs before and after Tocilizumab treatment. -

Whither Radioimmunotherapy: to Be Or Not to Be? Damian J
Published OnlineFirst April 20, 2017; DOI: 10.1158/0008-5472.CAN-16-2523 Cancer Perspective Research Whither Radioimmunotherapy: To Be or Not To Be? Damian J. Green1,2 and Oliver W. Press1,2,3 Abstract Therapy of cancer with radiolabeled monoclonal antibodies employing multistep "pretargeting" methods, particularly those has produced impressive results in preclinical experiments and in utilizing bispecific antibodies, have greatly enhanced the thera- clinical trials conducted in radiosensitive malignancies, particu- peutic efficacy of radioimmunotherapy and diminished its toxi- larly B-cell lymphomas. Two "first-generation," directly radiola- cities. The dramatically improved therapeutic index of bispecific beled anti-CD20 antibodies, 131iodine-tositumomab and 90yttri- antibody pretargeting appears to be sufficiently compelling to um-ibritumomab tiuxetan, were FDA-approved more than a justify human clinical trials and reinvigorate enthusiasm for decade ago but have been little utilized because of a variety of radioimmunotherapy in the treatment of malignancies, particu- medical, financial, and logistic obstacles. Newer technologies larly lymphomas. Cancer Res; 77(9); 1–6. Ó2017 AACR. "To be, or not to be, that is the question: Whether 'tis nobler in the pembrolizumab (anti-PD-1), which are not directly cytotoxic mind to suffer the slings and arrows of outrageous fortune, or to take for cancer cells but "release the brakes" on the immune system, arms against a sea of troubles, And by opposing end them." Hamlet. allowing cytotoxic T cells to be more effective at recognizing –William Shakespeare. and killing cancer cells. Outstanding results have already been demonstrated with checkpoint inhibiting antibodies even in far Introduction advanced refractory solid tumors including melanoma, lung cancer, Hodgkin lymphoma and are under study for a multi- Impact of monoclonal antibodies on the field of clinical tude of other malignancies (4–6). -

Federal Register/Vol. 83, No. 31/Wednesday, February 14, 2018
Federal Register / Vol. 83, No. 31 / Wednesday, February 14, 2018 / Notices 6563 Wireless Telecommunications Bureau Signed: DEPARTMENT OF HEALTH AND and Wireline Competition Bureau (the Dayna C. Brown, HUMAN SERVICES Bureaus) may implement, and (3) certify Secretary and Clerk of the Commission. Centers for Disease Control and its challenge. The USAC system will [FR Doc. 2018–03166 Filed 2–12–18; 4:15 pm] validate a challenger’s evidence using Prevention BILLING CODE 6715–01–P an automated challenge validation [CDC–2018–0004; NIOSH–233–B] process. Once all valid challenges have been identified, a challenged party that NIOSH List of Antineoplastic and Other chooses to respond to any valid FEDERAL RESERVE SYSTEM Hazardous Drugs in Healthcare challenge(s) may submit additional data Settings: Proposed Additions to the via the online USAC portal during the Change in Bank Control Notices; NIOSH Hazardous Drug List 2018 established response window. A Acquisitions of Shares of a Bank or AGENCY: Centers for Disease Control and challenged party may submit technical Bank Holding Company information that is probative regarding Prevention, HHS. ACTION: Notice of draft document the validity of a challenger’s speed tests, The notificants listed below have available for public comment. including speed test data and other applied under the Change in Bank device-specific data collected from Control Act (12 U.S.C. 1817(j)) and transmitter monitoring software or, SUMMARY: The National Institute for § 225.41 of the Board’s Regulation Y (12 alternatively, may submit its own speed Occupational Safety and Health test data that conforms to the same CFR 225.41) to acquire shares of a bank (NIOSH) of the Centers for Disease standards and requirements specified by or bank holding company.