Phylogenetic Diversity of Arctic Vertebrate Herbivores
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Role of Diseases in Mass Mortality of Wood Lemmings (Myopus Schisticolor)
The role of diseases in mass mortality of wood lemmings (Myopus schisticolor) Sjukdomars roll i massutdöende av skogslämmel (Myopus schisticolor) Henrik Johansen Master’s thesis • 30 credits Swedish University of Agricultural Sciences, SLU Department of Wildlife, Fish, and Environmental Studies Forest Science programme Examensarbete/Master’s thesis, 2021:7 Umeå, Sweden 2021 The role of disease in mass mortality of wood lemming (Myopus schisticolor) Sjukdomars roll I massutdöende av skogslämmel (Myopus schisticolor) Henrik Johansen Supervisor: Frauke Ecke, Swedish University of Agricultural Science, Department of wildlife, Fish, and Environmental Studies Assistant supervisor: Magnus Magnusson, Swedish University of Agricultural Science, Department of wildlife, Fish, and Environmental Studies Examiner: Joris Cromsigt, Swedish University of Agricultural Science, Department of wildlife, Fish, and Environmental Studies Credits: 30 credits Level: Second cycle, A2E Course title: Master’s thesis in Forest Science, A2E - Wildlife, Fish, and Environmental Studies Course code: EX0840 Programme/education: Forest Science programme Course coordinating dept: Department of Wildlife, Fish, and Environmental Studies Place of publication: Umeå, Sweden Year of publication: 2021 Cover picture: Thomas Secher Jensen Title of series: Examensarbete/Master’s thesis Part number: 2021:7 Keywords: Wood lemming, Myopus schisticolor, Disease, Virus, Pathogens, Mass mortality, Orthohantavirus, Pan-orthohantavirus, Somatic index, Spleen index Swedish University of Agricultural Sciences Faculty of Forest Science Department of Wildlife, Fish, and Environmental Studies Publishing and archiving Approved students’ theses at SLU are published electronically. As a student, you have the copyright to your own work and need to approve the electronic publishing. If you check the box for YES, the full text (pdf file) and metadata will be visible and searchable online. -

DNA Metabarcoding of Stomach Contents
1 Shedding new light on the diet of Norwegian lemmings: DNA 2 metabarcoding of stomach content 3 4 Eeva M. Soininen 1*, Lucie Zinger 2,7 , Ludovic Gielly 2, Eva Bellemain 3,6 , Kari Anne Bråthen 1, 5 Christian Brochmann 3, Laura S. Epp 3, Galina Gussarova 3, Kristian Hassel 4, John-André 6 Henden 1, Siw T. Killengreen 1, Teppo Rämä 5, Hans K. Stenøien 4, Nigel G. Yoccoz 1, Rolf A. Ims 1 7 1 Department of Arctic and Marine Biology, University of Tromsø, NO-9037 Tromsø, Norway 8 2Laboratoire d’ECologie Alpine, UMR UJF-UdS-CNRS 5553, Université Joseph Fourier, 2233 9 rue de la Piscine, BP 53, 38041 Grenoble Cedex 9, France 10 3 National Centre for Biosystematics, Natural History Museum, University of Oslo, PO 11 Box 1172 Blindern, NO-0318 Oslo, Norway 12 4 Museum of Natural History, Norwegian University of Science and Technology, NO-7491 13 Trondheim, Norway 14 5 Tromsø University Museum, University of Tromsø, NO-9037 Tromsø, Norway 15 6Current address: Savoie Technolac, Batiment House Boat n7 12, allee du lac de Garde, BP 16 274, 73375 Le Bourget-du-Lac Cedex, France 17 7Current address: Laboratoire Evolution et Biodiversité Biologique, UMR 5174, Université 18 Paul Sabatier, 118 route de Narbonne, 31062 Toulouse Cedex 9, France 19 *Corresponding author e-mail: [email protected] , tel: +47 77620932, 20 fax: +47 77 64 63 33 1 21 Abstract 22 Lemmings are key herbivores in many arctic food webs and their population dynamics have 23 major impacts on the functioning of tundra systems. -

Prey Selection and Prey Handling in Two Raptors During the Breeding Season As Revealed by the Use of Video Monitoring
Norwegian University of Life Sciences Department of Ecology and Natural Resource Management (INA) Master Thesis 2015 30 credits Prey selection and prey handling in two raptors during the breeding season as revealed by the use of video monitoring Byttedyrvalg og byttedyrhåndtering hos to rovfugler i hekkeperioden, avslørt ved bruk av videoovervåking Ida Dihle A C K N O W L E D G E M E N T S First of all, I would like to thank Fylkesmannen i Oppland for the financial support. I would like to give a huge thanks to my brilliant supervisor Geir A. Sonerud who has spend hours identifying prey, helping with the statistical analyses and thoroughly reviewing my drafts. I am also very grateful to supervisor Ronny Steen, for all his effort regarding all stages of the fieldwork, his technical support throughout the process and providing helpful comments on my drafts. I would like to direct a big thanks to Vidar Selås, who contributed with his expertise when identifying prey. I am very grateful for the effort, inspiration and company during fieldwork given by Helge Grønnlien. The last weeks of recordings was made possible because of him. Dag S. Rusten also deserves a thanks, who along with Ronny Steen and Helge Grønnlien installed the cameras at the nests. My friends, Lene M. Rabben in particular, and family has been a big help throughout the process. I would like to thank my parents for providing equipment, and joining me in field, and Thea Dihle for punching numbers in the less inspiring periods of the writing process. -

Siberian Coast & Polar Bears of Wrangel Island, Russia
Siberian Coast & Polar Bears of Wrangel Island, Russia Aboard the Spirit of Enderby July 24–August 6, 2013 Tuesday / Wednesday July 23 / July 24 – Anadyr, Russia Arrival Over several days, North American trip participants slowly began to arrive in Nome, Alaska for our charter flights to Anadyr, Russia. Participants from other parts of the world made their way to Anadyr through Moscow. Altogether, passengers included passport holders from 11 countries. Many people had flown into Nome several days early, rented cars, and driven the local roads in search of accessible musk oxen in the meadows near town, Pacific and red-throated loons in small tundra ponds, and gulls, ducks and shorebirds along the coast. On July 23, most of our group consolidated their luggage in our Nome hotel lobby and had it loaded on a truck to be taken to our charter airline hangar, while we followed in a bus with our camera gear. Thick fog was drifting along the coast and, when we reached the hanger, we were informed the Nome Airport was closed to inbound air traffic. That posed no real problem for us because our small airplanes were already at the hangar in Nome, on stand-by and readily available, and, since the fog was intermittent, it seemed certain we would have almost no problem departing to Russia. A breakfast buffet was arranged in the hangar and we were regaled by local Nome personality Richard Beneville—who also organized our land-based transportation—with his tales of moving to Nome following his life as a theater actor in New York City and anecdotes of his decades-long life in hardscrabble Nome. -

Relating the 4-Year Lemming Population Cycle to a 3.8-Year Lunar Cycle and ENSO
Canadian Journal of Zoology Relating the 4-year lemming population cycle to a 3.8-year lunar cycle and ENSO Journal: Canadian Journal of Zoology Manuscript ID cjz-2018-0266.R3 Manuscript Type: Article Date Submitted by the 01-Jun-2019 Author: Complete List of Authors: Archibald, Herbert; Retired, ; None, None Is your manuscript invited for consideration in a Special Not applicable (regular submission) Issue?: Draft population cycle, lemming (Lemus spp. and Dicrostonyx spp.), zeitgeber, Keyword: lunar cycle, ENSO, Southern Oscillation Index (SOI) https://mc06.manuscriptcentral.com/cjz-pubs Page 1 of 37 Canadian Journal of Zoology Relating the 4-year lemming population cycle to a 3.8-year lunar cycle and ENSO Archibald, H.L. 632 Tee Hi Place, Medford, Wisconsin 54451 USA Correspondence: [email protected] Tel: 1-715-748-6536 Archibald, H. L. 2019. Relating the 4-year lemming population cycle to a 3.8-year lunar cycle and ENSO Draft 1 https://mc06.manuscriptcentral.com/cjz-pubs Canadian Journal of Zoology Page 2 of 37 Abstract Reported peak years of lemming (Lemmus spp. and Dicrostonyx spp.) and Arctic fox (Vulpes lagopus (Linnaeus, 1758)) abundance were compiled from the literature for 12 locations spanning 127 years. The mean period of the 34 reported lemming and Arctic fox cycles from 1868 to 1994 was 3.8 years, suggesting that the period of the 4-year cycle is actually 3.8 years. Peak population years were predicted using a simple model based on a 3.8-year lunar cycle. For nearly 130 years, reported years of peak abundance of lemmings and Arctic foxes were significantly correlated with and have persistently stayed in phase with predicted peak years of abundance. -

A-Nadachowski.Vp:Corelventura
Acta zoologica cracoviensia, 58(2): 181-194, Kraków, 31 December, 2015 Ó Institute of Systematics and Evolution of Animals, Pol. Acad. Sci., Kraków doi:10.3409/azc.58_2.181 NewLatePleistocenefaunalassemblagesfromPodhaleBasin, WesternCarpathians,Poland:preliminaryresults AdamNADACHOWSKI and Pawe³ VALDE-NOWAK Received: 05 October 2015. Accepted: 21 December 2015. Available online: 30 December 2015. NADACHOWSKI A., VALDE-NOWAK P. 2015. New Late Pleistocene faunal assem- blages from Podhale Basin, Western Carpathians, Poland: preliminary results. Acta zool. cracov., 58(2): 181-194. Abstract. Interdisciplinary studies in Ob³azowa Cave undertaken near its western entrance and in a new archaeological site in Cisowa Rock yielded finely layered sedimentary se- quences with an abundant fossil fauna of mainly small vertebrates and scarce archaeologi- cal finds. In this paper we present a preliminary palaeoenvironmental and palaeoclimatic reconstruction of the sequences dated around MIS 2/MIS 1 boundary on the basis of small mammal assemblages. The faunal changes appear to be gradual in spite of abrupt climatic changes expected to occur at that time. The environmental and faunal results are discussed with other sequences of similar age in Poland. Key words: fossil fauna, habitat and climate change, steppe-tundra, radiocarbon dating, Ob³azowa Cave. * Adam NADACHOWSKI, Institute of Systematics and Evolution of Animals Polish Acad- emy of Sciences, S³awkowska Str. 17, 31-016 Kraków, Poland. E-mail: [email protected] Pawe³ VALDE-NOWAK, Institute of Archaeology, Jagiellonian University, Go³êbia Str. 11, 31-007 Kraków, Poland. E-mail: [email protected] I.INTRODUCTION The Podhale Basin, also known as Orawa-Nowy Targ Basin, is located in the Western Carpathians, south Poland. -

Putorana Plateau Russian Federation
PUTORANA PLATEAU RUSSIAN FEDERATION This basalt dome in northern Siberia above the Arctic Circle is at the northern end of a vast basalt effusion which occurred at the Permian-Triassic boundary 250 million years ago. As a result of tectonic uplift it is deeply dissected by canyons with slopes eroded into giant steps. It lies between northern taiga and Arctic tundra on the border between a humid cyclonic and a dry continental climatic regime and its flora is therefore relatively rich for its latitude. The area supports a wide range of Arctic ecosystems. It is also notable for many lakes and waterfalls, for an isolated population of Putorana snow sheep, and for the major avian flyway and reindeer migration route which cross it. COUNTRY Russian Federation NAME Putorana Plateau NATURAL WORLD HERITAGE SITE 2010: Inscribed on the World Heritage List under Natural Criteria vii and ix. STATEMENT OF OUTSTANDING UNIVERSAL VALUE The UNESCO World Heritage Committee adopted the following Statement of Outstanding Universal Value at the time of inscription: Brief Synthesis This site coincides with the area of the Putoransky State Nature Reserve, and is located in the central part of the Putorana Plateau in northern Central Siberia. It is situated about 100 km north of the Arctic Circle. The part of the plateau inscribed on the World Heritage List harbours a complete set of subarctic and arctic ecosystems in an isolated mountain range, including pristine taiga, forest tundra, tundra and arctic desert systems, as well as untouched cold-water lake and river systems. A major reindeer migration route crosses the property, which represents an exceptional, large-scale and increasingly rare natural phenomenon. -

Norway's Environmental Targets
Norway’s Environmental Targets Action for the environment 3 ————————— Priority areas and tools Useful for achieving our goals 4 websites: Priority areas www.miljo.no 01 Living seas and coastal environments 6 www.environment.no 02 Healthy lakes and rivers 10 www.klif.no 03 Rich and varied wetlands 14 www.dirnat.no 04 Forest diversity 18 www.npolar.no 05 Spectacular mountain landscapes 22 www.ra.no 06 A valuable cultural heritage 26 www.statkart.no 07 A good urban environment 30 08 The great outdoors 34 ————————— 09 An unpolluted environment 38 10 Clean air 42 11 A stable climate 46 Instruments Knowledge and geographical information 50 Legislation and planning procedures 52 International cooperation 54 Norway’s Environmental Targets was published in Norwegian in 2011. This translated version contains some updates and corrections, but has not been completely revised. 7U\NN$QGYRUG*UDILVN$6 Action for the environment Thirty years ago, acid rain was killing fish People have always left evidence of their in Norwegian rivers. Now, both salmon and activities behind them, altering the environ- trout are back again in many rivers thanks to ment for better or worse. Our cultural herit- concerted national and international efforts age does more than tell our history – it is also to cut emissions. part of a dynamic contemporary environ- ment. But it, too, is vulnerable: once build- Norway’s nature reaches from the deepest ings and cultural landscapes are destroyed seabed to the topmost mountain peaks, or removed, they are lost for ever. and from the thickest forest to the highest waterfall. -
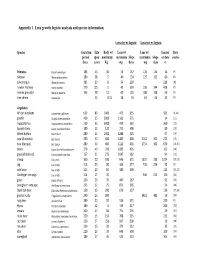
Appendix 1. Lens Growth Logistic Analysis and Species Information
Appendix 1. Lens growth logistic analysis and species information. Lens dry wt logistic Lens wet wt logistic Species Gestation Life Body wt Lens wt Lens wt Lenses Data period span maximum maximum Slope maximum Slope or data source days years Kg mg days mg days n Primates Papio hamadryas 183 45 30 55 167 178 124 16 PS baboon Macaca fascicularis 180 38 5 40 130 125 162 60 PS cynomolgus Alouatta caraya 185 20 8 34 220 228 [8] howler monkey Homo sapiens 270 115 5 45 359 198 244 608 PS human prenatal Macaca mulatta 165 38 12 60 125 188 108 50 PS tree shrew Tupaia glis 12 0.15 28 76 63 63 32 PS Ungulates African elephant Loxodonta a africana 659 80 5000 475 935 561 [9,10] giraffe Giraffa camelopardalis 460 35 2000 1163 571 34 [11] hipppotamus Hippopotamus amphibius 250 55 3000 410 362 360 [12] Spanish Ibex Capra pyrenaica Schinz 180 12 120 711 508 80 [13] Wood buffalo Bison bison 280 25 1000 1288 525 97 [14] cow (Australia) Bos taurus 280 30 600 1483 400 3162 302 231 [15] cow (Europe) Bos taurus 280 30 400 1122 401 2754 302 630 [16,17] zebra Equus burchelli antiquorum 370 40 350 1085 436 102 [18] gnu (wildebeest) Connochaetes taurinus 257 21 275 1047 362 94 [19] sheep Ovis aries 160 20 100 646 311 1622 239 1224 [20,21] pig Sus scrofa 115 25 80 364 277 736 254 92 PS wild boar Sus scrofa 115 20 84 389 309 113 [22,23] Gottingen minipigs Sus scrofa 114 17 35 646 211 100 [24] goat Capra hircus L 150 20 70 447 267 91 [25] pronghorn antelope Antilocapra americana 235 12 75 871 365 24 [26] black tail deer Odocoileus hemionus columbianus 150 15 200 -

Ice Age Animal Factsheets
Ice Age Animal Factsheets Scientists have found frozen woolly mammoths in Height: 3m (shoulder) cold places, like Russia. Weight: 6000 kg Some of these mammoths Diet: Herbivore were so complete that you Range: Eurasia & can see their hair and N. America muscles! WOOLLY MAMMOTH HERBIVORES: MEGAFAUNA The word “megafauna” means “big animal”. Many animals in the Ice Age were very large compared with creatures living today. WOOLLY RHINOCEROS Woolly rhinos were plant- eaters. They used their Height: 2m (shoulder) massive horns to scrape Weight: 2700 kg away ice and snow from Diet: Herbivore plants so they could feed Range: Eurasia (like a giant snow plough)! In Spain, there is a cave called Altamira which has Height: 2m (shoulder) beautiful Ice Age paintings Weight: 900 kg of bison. We also have Diet: Herbivore an Ice Age carv ing of a Range: Eurasia bison at Creswell Crags! STEPPE BISON HERBIVORES: COW-LIKE ANIMALS Aurochs and bison were large animals which looked a lot like bulls. Humans during the Ice Age would have hunted them for their meat and skins. Bison were one of the most popular The farm cows we have choicesAUROCHS of subject in Stone Age art. today are related to the Height: 1.8m (shoulder) aurochs. Scientists think Weight: 700 kg humans began to tame Diet: Herbivore aurochs around ten thousand Range: Eurasia & years ago. Africa The saiga antelope has a very unusual nose which Height: 0.7m (shoulder) helps them live in cold places. Breathing Weight: 40 kg through their nose lets Diet: Herbivore them warm up cold air! Range: Eurasia SAIGA ANTELOPE HERBIVORES: GRAZERS Grazers are animals which eat food on the ground, usually grasses or herby plants. -

A Global-Scale Evaluation of Mammalian Exposure and Vulnerability to Anthropogenic Climate Change
A Global-Scale Evaluation of Mammalian Exposure and Vulnerability to Anthropogenic Climate Change Tanya L. Graham A Thesis in The Department of Geography, Planning and Environment Presented in Partial Fulfillment of the Requirements for the Degree of Master of Science (Geography, Urban and Environmental Studies) at Concordia University Montreal, Quebec, Canada March 2018 © Tanya L. Graham, 2018 Abstract A Global-Scale Evaluation of Mammalian Exposure and Vulnerability to Anthropogenic Climate Change Tanya L. Graham There is considerable evidence demonstrating that anthropogenic climate change is impacting species living in the wild. The vulnerability of a given species to such change may be understood as a combination of the magnitude of climate change to which the species is exposed, the sensitivity of the species to changes in climate, and the capacity of the species to adapt to climatic change. I used species distributions and estimates of expected changes in local temperatures per teratonne of carbon emissions to assess the exposure of terrestrial mammal species to human-induced climate change. I evaluated species vulnerability to climate change by combining expected local temperature changes with species conservation status, using the latter as a proxy for species sensitivity and adaptive capacity to climate change. I also performed a global-scale analysis to identify hotspots of mammalian vulnerability to climate change using expected temperature changes, species richness and average species threat level for each km2 across the globe. The average expected change in local annual average temperature for terrestrial mammal species is 1.85 oC/TtC. Highest temperature changes are expected for species living in high northern latitudes, while smaller changes are expected for species living in tropical locations. -

Microevolution of Puumala Hantavirus in Its Host, the Bank Vole (Myodes Glareolus)
Microevolution of Puumala hantavirus in its host, the bank vole (Myodes glareolus) Maria Razzauti Sanfeliu Research Programs Unit Infection Biology Research Program Department of Virology, Haartman Institute Faculty of Medicine, University of Helsinki and the Finnish Forest Research Institute Academic dissertation Helsinki 2012 To be presented for public examination, with the permission of the Faculty of Medicine, University of Helsinki, in Lecture Hall 2, Haartman Institute on February the 24th at noon Supervisors Professor, docent Alexander Plyusnin Department of Virology, Haartman Institute P.O.Box 21, FI-00014 University of Helsinki, Finland e-mail: [email protected] Professor Heikki Henttonen Finnish Forest Research Institute (Metla) P.O.Box 18, FI-01301 Vantaa, Finland e-mail: [email protected] Reviewers Docent Petri Susi Biosciences and Business Turku University of Applied Sciences, Lemminkäisenkatu 30, FI-20520 Turku, Finland e-mail: [email protected] Professor Dennis Bamford Department of Biological and Environmental Sciences Division of General Microbiology P.O.Box 56, FI-00014 University of Helsinki, Finland e-mail: [email protected] Opponent Professor Herwig Leirs Dept. Biology, Evolutionary Ecology group Groenenborgercampus, room G.V323a Groenenborgerlaan 171, B-2020 University of Antwerpen, Belgium e-mail: [email protected] ISBN 978-952-10-7687-9 (paperback) ISBN 978-952-10-7688-6 (PDF) Helsinki University Print. http://ethesis.helsinki.fi © Maria Razzauti Sanfeliu, 2012 2 Contents