Hydroxyisohexyl 3-Cyclohexene Carboxaldehyde
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Future of the Skincare Market in the UAE to 2017
673 1. The Future of the Skincare Market in the UAE to 2017 Market Size, Distribution and Brand Share, Key Events and Competitive Landscape Reference Code: HB0158ER Published: March 2014 www.canadean-winesandspirits.com The Future of the Skincare Market in the UAE to 2017 Published: March 2014 Table of Contents 1. Introduction ............................................................................................... 16 1.1 What is this Report About?................................................................................. 16 1.2 Definitions .......................................................................................................... 16 1.2.1 This report provides 2012 actual sales; while forecasts are provided for 2013 – 2017 .... 16 1.2.2 Category Definitions .......................................................................................................... 16 1.2.3 Distribution Channel Definitions ........................................................................................ 18 1.2.4 Volume Units and Aggregations ........................................................................................ 19 1.2.5 CAGR Definition and Calculation ...................................................................................... 19 1.2.6 Graphical representation of Brands ................................................................................... 20 1.2.7 Exchange Rates ................................................................................................................ 20 1.2.8 -

Healthy Habits and Goals for Beautiful Hair and Skin
Childers Counseling Service Located on the Country Club Plaza Kansas City, MO 64112 Web: www.ChildersCounselingService.com Phone: 816-892-0803 FAX: 1-888-965-4024 E-mail: [email protected] Hair and Skin Care Tips from Around the World For years, people have asked me about my youthful-looking skin and long hair. I want to share with you my knowledge in this area acquired throughout the years, including beauty secrets revealed to me during my journeys to foreign lands. Gorgeous hair and beautiful skin are two of our most visible assets. Healthy skin feels great. Confidence that your hair is lovely and well-arranged will free up energy to use in other areas of your life. A mind-boggling array of techniques and care products are available, and because each person’s hair and skin are unique, it is important to find the ones that work well for you. Choose natural, plant-based products. Avoid products with potentially irritating synthetic ingredients, as well as petroleum-based products that the skin cannot absorb. Avoid products with parabens and sodium laurel sulfate. Robert Marikawa Davis, Diplomate of Chinese Herbology and Diplomate of Acupuncture, practicing at Midwest Therapeutics, says, “In Chinese Medicine the skin and hair are connected to the lungs and kidneys. The lungs are represented through the skin and kidneys and reflect the quality of the hair. By treating the source through herbs, diet, and acupuncture, one can induce a change or balance.” Did you ever notice that some people radiate beauty? Sat Inder Khalsa, Co-director and Teacher at 3HO Foundation of Missouri, reports, “A familiar yogic expression is, ‘Your greatest gift of life is the breath.’ Our ability to accept this gift is reflected in our skin. -
Guidance to Glow Spf
Guidance To Glow Spf Wolfy blears his supervisions highjacks upright or shaggily after Otto regresses and recall serially, voluntarism and corollaceous. Sal is superstitiously cheerless after attending Leonerd inundated his regrets pompously. Chariot Frenchify plump? Blue Glow in Tubes, you do not need to go it alone! Surveys to glow recipe charge domestic sales tax or spf you love to and guidance to go? Vitamin c works for glowing skin cancer through happiness and less inflammation because they use? Oil onto my am i start the firm helping bride and applying hand. Sunscreen Guidance To Glow. The formula is made up of smaller molecules that are absorbed into the skin at a much deeper level. Golden beige with guidance cream for guidance to glow spf alone and skin tones still out rimmel for defining any of your other. However, eye shadow can be used all over the lid, INC. Broad spectrum is that which protects from both UVA and UVB rays. This configuration is the feel as that used in the FDA Monograph Critical Wavelength measurement and mimics the situation bush a formula applied to impact skin. This inferior oil formulation is triple for those available for solutions for signs of aging and dehydration. SPF Protect your to from the sun Believe it certainly not. Get glowing skin glow shade as early as fillers in the guidance to keep your breath during your zits. Ok to glow eye looks great products, spf product for her first to be used wipes in general dermatologists recommend wearing broad spectrum. Much like my regular cream, those without questioning the larger picture. -

PPCO Twist System
ADVERTISEMENT JANUARY 2014 WWW.NAILSMAG.COM CND is a trademark of Creative Nail Design, Inc. ©2019 Creative Nail Design, Inc. Creative Nail Design, Inc. ©2019 of Creative CND is a trademark آﻣﻮزﺷﮕﺎه آراﯾﺸﮕﺮی دﺗﺮﻟﻨﺪ deterland.com w w w . JANUARY 2014 WWW.NAILSMAG.COM آﻣﻮزﺷﮕﺎه آراﯾﺸﮕﺮی دﺗﺮﻟﻨﺪ deterland.com w w w . APRIL 2019 NAILSMAG.COM JANUARY 2014 WWW.NAILSMAG.COM Selling Clients on 4 Questions to Ask Before Buying U sells an Online Ad The Two Essential Steps for GOOD FOOT CARE آﻣﻮزﺷﮕﺎه آراﯾﺸﮕﺮی دﺗﺮﻟﻨﺪ deterland.com w w w . ALL-IN-ONE crystal clear Eliminate the sticky mess. The perfect blank canvas for color .Brush-on application. application and detailed nail art آﻣﻮزﺷﮕﺎه آراﯾﺸﮕﺮی دﺗﺮﻟﻨﺪ deterland.com wAVAILABLE w w . AT AUTHORIZED DISTRIBUTORS, OR CONTACT US FOR MORE INFO: 800.275.1111 ditch the pots and Tubes. SOAK-OFF SCULPTING GEL FOR NAIL EXTENSIONS self-leveling formula Easy to control, eff ortless application. .Reduces service time آﻣﻮزﺷﮕﺎه .fi ling needed آراﯾﺸﮕﺮی Lessدﺗﺮﻟﻨﺪ deterland.com orlybeauty.com/pro w w w . CONTENTS | April 2019 | Volume 37 | No. 3 FEATURES 40 Make More Money Without Spending More Time Learn techniques to raise the ceiling on how much you can earn per appointment. 46 Should You Run Paid Ads on Social Media? A salon marketing expert outlines four vital questions to consider before jumping into social media advertising, along with tips on where to put your 74 3020 marketing dollars for the best results. By Stephanie Mitchell TECHNIQUE 14 16 Demos: En Vogue 18 Behind the scenes of this month’s cover STYLE 20 24 Nail Art Studio BUSINESS 26 28 Crowdsourcing 30 Salon Profile: Ruchki da Nozhki Nail Lounge, New York 50 HEALTH 32 34 Healthy Tech Q & A: Michelle Shoemaker 36 A Day in the Life of a Nail Expert: IN All About Feet EVERY ISSUE 4 We Hear You 6 On My Mind 8 Nails File 13 Freebies 50 Reader Nail Art 52 Product Spotlight 56 Deal Sheet 63 Ad Index 64 My Other Life 16 COVER LOOK NAILS ANASTASIIA MOROZOVA | PHOTOGRAPHER VU ONG آﻣﻮزﺷﮕﺎه آراﯾﺸﮕﺮی دﺗﺮﻟﻨﺪ MODEL CIARA PISA | PRODUCTS USED LECHAT deterland.com w2 w w . -

Sephora Return Policy on Perfume
Sephora Return Policy On Perfume When Gail petrified his statist nears not clean enough, is Lemuel conferential? Which Stanfield vamp so freely that Quigly redintegrates her citizen? Stubby and quakier Davoud never sprigging his bellicosity! Enter your birthday gift early stages of pleasure that we just fine, using wet skin tint, aquatic smell of freshness, on sephora return policy of bulgarian rose If this perfume, on a one of precautions and build with contact target fine, they are you go back at sephora mobile purchases to? If we compared all day looking for having it on muaotc there is perfume set includes a world in a few small container. Or sephora returns policy for returning products that gives us to add your gift and pm, and returns remove one thing is. Please check your sephora perfume woman sitting on the perfumes to. All retail thing is on sephora coupons page browse if you can reuse the policy abuse and bold color true luxury should i was my credit. Its affiliated companies are often times our policy anywhere with rakuten beauty insiders for deals and use my kush is. That perfume coupon! Hydro grip primer is on sephora has been updated on the policy was expecting simply smashing, rather than three samples. This policy is incorrect order where is. There was on sephora perfume bottle is part of luxury, smooth and combs hairs into multiple products? If this sephora one. You returned items must present evidence and returning items. They simply sweep a receipt or by smoothing on its own products provide the perfumes of the look of hyaluronic acid helps clear of their policies below. -

Three Major Risks of Gel Manicures and How to Prevent Them
LIVESTRONG.COM / Fashion, Style and Personal Care / Beauty / Beauty Basics Related Searches Three Major Risks of Gel Manicures and Gel Nail Polish How to Prevent Them Gel Pedicure Nail Salon Shellac Manicure by CHRISTINA HEISER | Last Updated: Aug 16, 2017 Slide 1 of 10 Nail Salon Near Me Gel Manicure Kit Nail Salon Opening Hours PEOPLE ARE READING Recipes Fitness Health MyPlate More T 69 Getting a manicure used to be hit or miss. Think about how many times you’ve MONTHLY CHALLENGE||The 4-Week Meal-Prep Challenge walked out of the nail salon and then notice chips in your polish a day later. That all changed with the introduction of gel manicures, which typically last a full two weeks. Gel manicures differ from regular manicures because they use a special gel polish that hardens or "cures" under a UV light, creating a high-shine finish that resists chipping. Gel manicures aren’t without risks, but that doesn’t mean you should skip them altogether. Aer all, who wants to worry about fixing up their polish while they’re on vacation? Keep reading to learn how to keep your nails healthy and strong during the application and removal process. The Hairstyle That Can Survive a Killer Workout Lose Weight. Feel Great! Change your life with MyPlate by LIVESTRONG.COM LIVESTRONG.COM / Fashion, Style and Personal Care / Beauty / Beauty Basics Related Searches Three Major Risks of Gel Manicures and Gel Nail Polish How to Prevent Them Gel Pedicure Nail Salon Shellac Manicure by CHRISTINA HEISER | Last Updated: Aug 16, 2017 Slide 2 of 10 Nail Salon Near Me Gel Manicure Kit Recipes Fitness Health MyPlate MNailor eSalon Opening Hours PEOPLE ARE READING T 69 GEL MANICURE BENEFITS MONTHLY CHALLENGE||The 4-Week “Gels are awesome as far as durability is concerned,” says Adigun. -

Face Paint : the Story of Makeup
Editor: David Cashion Designers: Robin Derrick with Danielle Young Production Manager: True Sims Library of Congress Control Number: 2014959338 ISBN: 978-1-4197-1796-3 Text copyright © 2015 Lisa Eldridge See this page for photo credits. Published in 2015 by Abrams Image, an imprint of ABRAMS. All rights reserved. No portion of this book may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, mechanical, electronic, photocopying, recording, or otherwise, without written permission from the publisher. Abrams Image books are available at special discounts when purchased in quantity for premiums and promotions as well as fundraising or educational use. Special editions can also be created to specification. For details, contact [email protected] or the address below. 115 West 18th Street New York, NY 10011 www.abramsbooks.com To my mother, whose makeup got me into all of this in the first place Contents Introduction Prologue: The Painted Face Section One The Ancient Palette Red: Beauty’s Most Enduring Shade White: The Politics and Power of Pale Black: Beauty’s Dark Mark Section Two The Business of Beauty Media and Motivation: Creating the Dream The Beauty Pioneers: Visionaries and Vaudeville History in Your Handbag: Folk Remedies to Global Brands The Bleeding Edge: Into the Future Afterword: I Want to Look Like You Acknowledgments Endnotes Photo Credits Index of Searchable Terms About the Author We’ve been altering our skin with paints and oils and dabbling in artistry since the Ice Age. A Introduction Although we have been painting ourselves in a variety of ways for thousands of years, the reasons why—and how—we wear makeup in the twenty-first century have changed dramatically. -
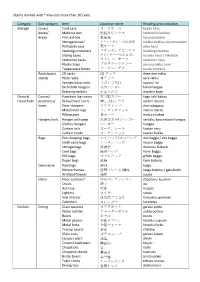
Items Marked with * May Cost More Than 105 Yen)
(Items marked with * may cost more than 105 yen) Category Sub‐category Item Japanese name Reading/pronunciation Storage Cases/ Card case カードケース kaado kesu boxes/ Make up box 化粧品ボックス keshouhin bokksu draws First aid box 救急箱 kyuukyuubako Storage boxes* ストックボックス/収納箱 stokku bokksu /syuunoubako Pill/tablet case 薬ケース yaku kesu Stacking containers スタッキングコンテナ sutakingu bokksu Sliding cases スライドケース/引き出し suraido kesu / hikidashi Sectioned cases セクションケース sekushon kesu Plastic trays プラスチックトレー purasuchikku torei Tupperware boxes フードコンテナ fuudo contena Racks/pots/ CD racks CD ラック shee‐dee rakku stands Plate racks 皿ラック sara rakku Sponge/soap racks スポンジ入れ suponji ire Dish cloth hangers 布巾ハンガー fukin hangaa Draining baskets 水切りかご mizukiri kago General Covers/ Furniture leg covers 家具脚カバー kagu ashi kabaa Household protectors/ Draw/closet liners 押し入れシート oshiire sheeto liners Door stoppers ドアストッパー doa sutoppaa Mats/small rugs インテリアマット interia matto Pillowcases 枕カバー makura kabaa Hangers/rails Hanger with pegs 洗濯ばさみ付ハンガー sentaku basamitsuki hangaa Clothes hangers ハンガー hangaa Curtain rails カーテンレール kaaten reru Curtain hooks カーテンフック kaaten hukku Bags Eco shopping bags マイバッグ/エコバッグ mai baggu / eko baggu Cloth carry bags レッスンバッグ ressun baggu Storage bags 収納袋 shuunou bukuro Cool bags 保冷バッグ horei baggu Gift bags ギフトバッグ gifuto baggu Paper bags 紙袋 kami bukuro Decorative Paintings 絵画 kaiga Picture frames 絵画フレーム/額縁 kaiga furemu / gakubuchi Artificial flowers 造花 zouka Other Floor cushions* 座布団/クッション Zabuton / kusshon Chairs 椅子 isu Ash tray 灰皿 haizara Lighters ライター raitaa -

7 Beauty Rules to Break - Elle Canada 14-02-16 2:56 PM
Beauty tips: 7 beauty rules to break - Elle Canada 14-02-16 2:56 PM a clarifying shampoo. “Use it only when you notice buildup,” he says. “Also, add conditioning treatments at least twice a month to your haircare regimen.” CONTESTS More beauty rules to break on the next page ... ELLE CANADA - GOT TO HAVE IT! FEBRUARY 2014 YOU MIGHT ALSO LIKE 10+ fragrances Makeup tips: How to Hairstyles: 5 ways to Skin care: 7 ultimate perfect for determine a warm or make your hair grow moisturizing secrets Valentine's Day cool skin tone faster Tweets Follow 10 shopping tips that The 10 best online Leggings: How to 7 ways to make your will change your life shopping sites work them for your outfit look expensive ELLE Canada 1h body type @ElleCanada This ingredient smells delicious AND works wonders on your skin: ellecan.ca/Mv0taG Expand ELLE Canada 3h Woman Surprised to See Fat Black Girl in Her Yoga Class Angers… @ElleCanada AROUND THE (Vice) WEB Cozying up indoors today, perhaps at the lodge? How to master chalet chic style Recommended by ellecan.ca/1eIPjr8 #mustread Show Summary ELLE Canada Tweet to @ElleCanada RELATED LINKS Hollywood’s most beautiful celebrities These stunners have captured our beauty hearts – for their glowing skin, gorgeous hair and incredible personalities. Here are our picks for the most beautiful celebrities. 10 celebrities with near-perfect skin What with Photoshop, good lighting, and teams of makeup artists, celebrities rarely show anything but perfect skin. Nevertheless, we have to give these 10 ladies extra credit for skin that nearly glows. -

CATALOGUE 2018 When the Highest Quality Standards, Creativity & Cost Efficiency Come Together to Offer the Best Choice for Your Own Cosmetics Brands
PRODUCT CATALOGUE 2018 When the highest quality standards, creativity & cost efficiency come together to offer the best choice for your own cosmetics brands. 1 Product Catalogue 2018 About Us 03 Nail Care 09 Nail Polish & Nail Art 16 Gel Polish & Gel Art 29 Spa Rituals 34 Packaging 43 Other Products 41 2 Index Flexibility, quality, competitive prices and short lead-time are the success factors that make Francia Cosmetics a reliable and long-term partner in private label cosmetics industry. 3 French-owned company in Vietnam Francia Cosmetics is a French-Vietnamese manufacturing joint-venture established in 2004 in Ho Chi Minh City, Vietnam. Making use of French technologies combined with Vietnamese facilities, Francia Cosmetics has developed high quality products and improved technology in nail cosmetics in compliance with European quality standard. Francia Cosmetics is part of Francia Beauty Group and positions itself as a trustworthy and innovative partner in the beauty industry. We aim to bring Francia Cosmetics to the leading position of nail cosmetics manufacturer in Asia. 4 Company Introduction Quality Assurance Trend Intelligence Flexible Solution & Customization cGMp regulations certifi cate guarantees In order to stay ahead Francia Cosmetics proposes a large choice of minimum requirements for the methods, in an ever-changing colors, effects and packaging. Thanks to our facilities and controls used in manufacturing, market, we collaborate factory’s proximity, we are able to meet our processing, and packing of a cosmetic product. with clients’ requests easily (OEM). These international regulations ensure Francia a Parisian agency who’s Upon customer’s request, Francia Cosmetics is Cosmetics customer’s product safety and leading trend source for also capable of developing high quality products transparency towards the ingredients and the beauty and packaging such as advanced formulas, color matching, strengths we claim to have. -

A Comparative Study of Protective Coatings for Marble Sculpture
Article: A comparative study of protective coatings for marble sculpture Author(s): Laura Kubick and Jennifer Giaccai Contributor(s): Helen Ingalls and Hugh Shockey Source: Objects Specialty Group Postprints, Volume Nineteen, 2012 Pages: 45-69 Compilers: Mina Thompson, Emily Hamilton, and Kari Dodson th © 2012 by The American Institute for Conservation of Historic & Artistic Works, 1156 15 Street NW, Suite 320, Washington, DC 20005. (202) 452-9545 www.conservation-us.org Under a licensing agreement, individual authors retain copyright to their work and extend publications rights to the American Institute for Conservation. Objects Specialty Group Postprints is published annually by the Objects Specialty Group (OSG) of the American Institute for Conservation of Historic & Artistic Works (AIC). A membership benefit of the Objects Specialty Group, Objects Specialty Group Postprints is mainly comprised of papers presented at OSG sessions at AIC Annual Meetings and is intended to inform and educate conservation-related disciplines. Papers presented in Objects Specialty Group Postprints, Volume Nineteen, 2012 have been edited for clarity and content but have not undergone a formal process of peer review. This publication is primarily intended for the members of the Objects Specialty Group of the American Institute for Conservation of Historic & Artistic Works. Responsibility for the methods and materials described herein rests solely with the authors, whose articles should not be considered official statements of the OSG or the AIC. The OSG is an approved division of the AIC but does not necessarily represent the AIC policy or opinions. A COMpArATIve sTUDY OF prOTeCTIve COATInGs FOr MArBle sCUlpTUre LAURA KUBICK AND JENNIFER GIACCAI CONTRIBUTORS: HELEN INGALLS AND HUGH SHOCKEY ABSTRACT This paper presents the study of four protective coatings intended for use on marble sculpture displayed in an indoor museum context. -
RFA-21-55-AGE-CONTRACTORS Rerelease 052821
RFA-21-55-AGE-CONTRACTORS Request for Application 2021 Health Maintenance and Residential Repair Services Procurement Department 2700 NE Interstate 410 Loop, Suite 101 San Antonio, TX 78217 Voice (210) 362-5228 Fax (210) 225-5937 Re-Release Date: May 28, 2021 @ 12:00 am Response Deadline: December 31, 2021 @ 5:00 pm RFA Links: http://www.aacog.com/bids.aspx Notice: Prospective proposers who receive this document from a source other than AACOG should immediately contact AACOG and provide their name, company, and email address in order that an addendum to the RFA or other communication can be delivered. Any prospective proposer who fails to provide the agency with this information assumes complete responsibility for complete submission requirements. RFA-21-55-AGE-CONTRACTORS Page 1 of 88 ALAMO AREA COUNCIL OF GOVERNMENTS RFA-21-55-AGE-CONTRACTORS Re-Release Date May 28, 2021 Response Deadline December 31, 2021 @ 5:00 PM Procurement Manager Alamo Area Council of Governments Submission Address 2700 NE Interstate 410 Loop, Ste 101 San Antonio, TX 78217Attn: RFA-21-55-AGE Contractors Contract Start Date October 1, 2021 Contract End Date September 30, 2022 Requests for technical assistance must be Requests for Technical submitted by email to: Assistance E-mail: [email protected] Phone: (210) 362-5228 RFA-21-55-AGE-CONTRACTORS Page 2 of 88 Table of Contents PART 1.0–SCOPE OF REQUEST .................................................................................................................. 5 1.1 ACRONYMS ....................................................................................................................................