LEAF MONKEY, Trachypithecus Auratus Sondaicus, in the PANGANDARAN NATURE RESERVE, WEST JAVA, INDONESIA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Book of Abstracts IB2019
BOOK OF ABSTRACTS Université de la Réunion Campus du Moufia 2 Island Biology Third International Conference on Island Ecology, Evolution and Conservation 8-13 July 2019 University of La R´eunion Saint Denis, France Book of Abstracts Editors: Olivier Flores Claudine Ah-Peng Nicholas Wilding Description of contents This document contains the collection of abstracts describing the research works presented at the third international conference on island ecology, evolution and conservation, Island Biology 2019, held in Saint Denis (La R´eunion,8-13 July 2019). In the following order, the different parts of this document concern Plenary sessions, Symposia, Regular sessions, and Poster presentations organized in thematic sections. The last part of the document consists of an author index with the names of all authors and links to the corresponding abstracts. Each abstract is referenced by a unique number 6-digits number indicated at the bottom of each page on the right. This reference number points to the online version of the abstract on the conference website using URL https://sciencesconf.org:ib2019/xxxxxx, where xxxxxx is the reference of the abstract. 4 Table of contents Plenary Sessions 19 Introduction to natural history of the Mascarene islands, Dominique Strasberg . 20 The history, current status, and future of the protected areas of Madagascar, Steven M. Goodman............................................ 21 The island biogeography of alien species, Tim Blackburn.................. 22 What can we learn about invasion ecology from ant invasions of islands ?, Lori Lach . 23 Orchids, moths, and birds on Madagascar, Mauritius, and Reunion: island systems with well-constrained timeframes for species interactions and trait change, Susanne Renner . -

Five Hundred Plant Species in Gunung Halimun Salak National Park, West Java a Checklist Including Sundanese Names, Distribution and Use
Five hundred plant species in Gunung Halimun Salak National Park, West Java A checklist including Sundanese names, distribution and use Hari Priyadi Gen Takao Irma Rahmawati Bambang Supriyanto Wim Ikbal Nursal Ismail Rahman Five hundred plant species in Gunung Halimun Salak National Park, West Java A checklist including Sundanese names, distribution and use Hari Priyadi Gen Takao Irma Rahmawati Bambang Supriyanto Wim Ikbal Nursal Ismail Rahman © 2010 Center for International Forestry Research. All rights reserved. Printed in Indonesia ISBN: 978-602-8693-22-6 Priyadi, H., Takao, G., Rahmawati, I., Supriyanto, B., Ikbal Nursal, W. and Rahman, I. 2010 Five hundred plant species in Gunung Halimun Salak National Park, West Java: a checklist including Sundanese names, distribution and use. CIFOR, Bogor, Indonesia. Photo credit: Hari Priyadi Layout: Rahadian Danil CIFOR Jl. CIFOR, Situ Gede Bogor Barat 16115 Indonesia T +62 (251) 8622-622 F +62 (251) 8622-100 E [email protected] www.cifor.cgiar.org Center for International Forestry Research (CIFOR) CIFOR advances human wellbeing, environmental conservation and equity by conducting research to inform policies and practices that affect forests in developing countries. CIFOR is one of 15 centres within the Consultative Group on International Agricultural Research (CGIAR). CIFOR’s headquarters are in Bogor, Indonesia. It also has offices in Asia, Africa and South America. | iii Contents Author biographies iv Background v How to use this guide vii Species checklist 1 Index of Sundanese names 159 Index of Latin names 166 References 179 iv | Author biographies Hari Priyadi is a research officer at CIFOR and a doctoral candidate funded by the Fonaso Erasmus Mundus programme of the European Union at Southern Swedish Forest Research Centre, Swedish University of Agricultural Sciences. -

Medicinal Practices of Sacred Natural Sites: a Socio-Religious Approach for Successful Implementation of Primary
Medicinal practices of sacred natural sites: a socio-religious approach for successful implementation of primary healthcare services Rajasri Ray and Avik Ray Review Correspondence Abstract Rajasri Ray*, Avik Ray Centre for studies in Ethnobiology, Biodiversity and Background: Sacred groves are model systems that Sustainability (CEiBa), Malda - 732103, West have the potential to contribute to rural healthcare Bengal, India owing to their medicinal floral diversity and strong social acceptance. *Corresponding Author: Rajasri Ray; [email protected] Methods: We examined this idea employing ethnomedicinal plants and their application Ethnobotany Research & Applications documented from sacred groves across India. A total 20:34 (2020) of 65 published documents were shortlisted for the Key words: AYUSH; Ethnomedicine; Medicinal plant; preparation of database and statistical analysis. Sacred grove; Spatial fidelity; Tropical diseases Standard ethnobotanical indices and mapping were used to capture the current trend. Background Results: A total of 1247 species from 152 families Human-nature interaction has been long entwined in has been documented for use against eighteen the history of humanity. Apart from deriving natural categories of diseases common in tropical and sub- resources, humans have a deep rooted tradition of tropical landscapes. Though the reported species venerating nature which is extensively observed are clustered around a few widely distributed across continents (Verschuuren 2010). The tradition families, 71% of them are uniquely represented from has attracted attention of researchers and policy- any single biogeographic region. The use of multiple makers for its impact on local ecological and socio- species in treating an ailment, high use value of the economic dynamics. Ethnomedicine that emanated popular plants, and cross-community similarity in from this tradition, deals health issues with nature- disease treatment reflects rich community wisdom to derived resources. -

Primates of the Southern Mentawai Islands
Primate Conservation 2018 (32): 193-203 The Status of Primates in the Southern Mentawai Islands, Indonesia Ahmad Yanuar1 and Jatna Supriatna2 1Department of Biology and Post-graduate Program in Biology Conservation, Tropical Biodiversity Conservation Center- Universitas Nasional, Jl. RM. Harsono, Jakarta, Indonesia 2Department of Biology, FMIPA and Research Center for Climate Change, University of Indonesia, Depok, Indonesia Abstract: Populations of the primates native to the Mentawai Islands—Kloss’ gibbon Hylobates klossii, the Mentawai langur Presbytis potenziani, the Mentawai pig-tailed macaque Macaca pagensis, and the snub-nosed pig-tailed monkey Simias con- color—persist in disturbed and undisturbed forests and forest patches in Sipora, North Pagai and South Pagai. We used the line-transect method to survey primates in Sipora and the Pagai Islands and estimate their population densities. We walked 157.5 km and 185.6 km of line transects on Sipora and on the Pagai Islands, respectively, and obtained 93 sightings on Sipora and 109 sightings on the Pagai Islands. On Sipora, we estimated population densities for H. klossii, P. potenziani, and S. concolor in an area of 9.5 km², and M. pagensis in an area of 12.6 km². On the Pagai Islands, we estimated the population densities of the four primates in an area of 11.1 km². Simias concolor was found to have the lowest group densities on Sipora, whilst P. potenziani had the highest group densities. On the Pagai Islands, H. klossii was the least abundant and M. pagensis had the highest group densities. Primate populations, notably of the snub-nosed pig-tailed monkey and Kloss’ gibbon, are reduced and threatened on the southern Mentawai Islands. -

Nonhuman Primates
Zoological Studies 42(1): 93-105 (2003) Dental Variation among Asian Colobines (Nonhuman Primates): Phylogenetic Similarities or Functional Correspondence? Ruliang Pan1,2,* and Charles Oxnard1 1School of Anatomy and Human Biology, University of Western Australia, Crawley, Perth, WA 6009, Australia 2Institute of Zoology, Chinese Academy of Sciences, Beijing 100080, China (Accepted August 27, 2002) Ruliang Pan and Charles Oxnard (2003) Dental variation among Asian colobines (nonhuman primates): phy- logenetic similarities or functional correspondence? Zoological Studies 42(1): 93-105. In order to reveal varia- tions among Asian colobines and to test whether the resemblance in dental structure among them is mainly associated with similarities in phylogeny or functional adaptation, teeth of 184 specimens from 15 Asian colobine species were measured and studied by performing bivariate (allometry) and multivariate (principal components) analyses. Results indicate that each tooth shows a significant close relationship with body size. Low negative and positive allometric scales for incisors and molars (M2s and M3s), respectively, are each con- sidered to be related to special dental modifications for folivorous preference of colobines. Sexual dimorphism in canine eruption reported by Harvati (2000) is further considered to be associated with differences in growth trajectories (allometric pattern) between the 2 sexes. The relationships among the 6 genera of Asian colobines found greatly differ from those proposed in other studies. Four groups were detected: 1) Rhinopithecus, 2) Semnopithecus, 3) Trachypithecus, and 4) Nasalis, Pygathrix, and Presbytis. These separations were mainly determined by differences in molar structure. Molar sizes of the former 2 groups are larger than those of the latter 2 groups. -

Deforestation of Primate Habitat on Sumatra and Adjacent Islands, Indonesia
Primate Conservation 2017 (31): 71-82 Deforestation of Primate Habitat on Sumatra and Adjacent Islands, Indonesia Jatna Supriatna1,2, Asri A. Dwiyahreni2, Nurul Winarni2, Sri Mariati3,4 and Chris Margules2,5 ¹Department of Biology, FMIPA Universitas Indonesia, Depok, Indonesia 2Research Center for Climate Change, Universitas Indonesia, Depok, Indonesia 3Postgraduate Program, Trisakti Institute for Tourism, Pesanggrahan, Jakarta, Indonesia 4Conservation International, Indonesia 5Centre for Tropical Environmental and Sustainability Science, College of Marine and Environmental Sciences, James Cook University, Cairns, Australia Abstract: The severe declines in forest cover on Sumatra and adjacent islands have been well-documented but that has not slowed the rate of forest loss. Here we present recent data on deforestation rates and primate distribution patterns to argue, yet again, for action to avert potential extinctions of Sumatran primates in the near future. Maps of forest loss were constructed using GIS and satellite imagery. Maps of primate distributions were estimated from published studies, museum records and expert opinion, and the two were overlaid on one another. The extent of deforestation in the provinces of Sumatra between 2000 and 2012 varied from 3.74% (11,599.9 ha in Lampung) to 49.85% (1,844,804.3 ha in Riau), with the highest rates occurring in the provinces of Riau, Jambi, Bangka Belitung and South Sumatra. During that time six species lost 50% or more of their forest habitat: the Banded langur Presbytis femoralis lost 82%, the Black-and-white langur Presbytis bicolor lost 78%, the Black-crested Sumatran langur Presbytis melalophos and the Bangka slow loris Nycticebus bancanus both lost 62%, the Lar gibbon Hylobates lar lost 54%, and the Pale-thighed langur Presbytis siamensis lost 50%. -

Llllllllllllllllllllllllllllll PUFF /MFN 9702
llllllllllllllllllllllllllllll PUFF /MFN 9702 Malayan Nature Joumal200l, 55 (1 & 2): 133 ~ 146 The Significance of Gynoecium and Fruit and Seed Characters for the Classification of the Rubiaceae CHRISTIAN(PUFF1 Abstract: This paper attempts to give a survey of the highly diverse situation found in the gynoecium (especially ovary) of the Rubiaceae (multi-, pluri-, pauci- and uniovulate locules; reduction of ovules and septa in the course of development, etc.). The changes that can take place after fertilisation (i.e., during fruit and seed development) are discussed using selected examples. An overview of the many diverse types of fruits and seeds of the Rubiaceae is presented. In addition, the paper surveys the diaspores (dispersal units) found in the family and correlates them with the morphological-anatomical situation. Finally, selected discrepancies between "traditional" classification systems of the Rubiaceae and recent cladistic analyses are discussed. While DNA analyses and cladistic studies are undoubtedly needed and useful, it is apparent that detailed (comparative) morphological-anatomical studies of gynoecium, fruits and seeds can significantly contribute to the solution of"problem cases" and should not be neglected, Future cladistic :work should, therefore, more generously include such data. INTRODUCTION The Rubiaceae, with approximately 11 ,000 species and more than 630 genera (Mabberley 1987, Robbrecht 1988), is one of the five largest families of angiosperms. Although centred in the tropics and subtropics and essentially woody, the family also extends to temperate regions and exhibits a wide array of growth forms, with some tribes having herbaceous, and even annual members. Not surprisingly, floral, fruit and seed characters also show considerable diversity. -
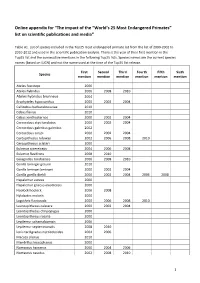
Online Appendix for “The Impact of the “World's 25 Most Endangered
Online appendix for “The impact of the “World’s 25 Most Endangered Primates” list on scientific publications and media” Table A1. List of species included in the Top25 most endangered primate list from the list of 2000-2002 to 2010-2012 and used in the scientific publication analysis. There is the year of their first mention in the Top25 list and the consecutive mentions in the following Top25 lists. Species names are the current species names (based on IUCN) and not the name used at the time of the Top25 list release. First Second Third Fourth Fifth Sixth Species mention mention mention mention mention mention Ateles fusciceps 2006 Ateles hybridus 2006 2008 2010 Ateles hybridus brunneus 2004 Brachyteles hypoxanthus 2000 2002 2004 Callicebus barbarabrownae 2010 Cebus flavius 2010 Cebus xanthosternos 2000 2002 2004 Cercocebus atys lunulatus 2000 2002 2004 Cercocebus galeritus galeritus 2002 Cercocebus sanjei 2000 2002 2004 Cercopithecus roloway 2002 2006 2008 2010 Cercopithecus sclateri 2000 Eulemur cinereiceps 2004 2006 2008 Eulemur flavifrons 2008 2010 Galagoides rondoensis 2006 2008 2010 Gorilla beringei graueri 2010 Gorilla beringei beringei 2000 2002 2004 Gorilla gorilla diehli 2000 2002 2004 2006 2008 Hapalemur aureus 2000 Hapalemur griseus alaotrensis 2000 Hoolock hoolock 2006 2008 Hylobates moloch 2000 Lagothrix flavicauda 2000 2006 2008 2010 Leontopithecus caissara 2000 2002 2004 Leontopithecus chrysopygus 2000 Leontopithecus rosalia 2000 Lepilemur sahamalazensis 2006 Lepilemur septentrionalis 2008 2010 Loris tardigradus nycticeboides -

REVIEW ARTICLE Agroecosystems and Primate Conservation in the Tropics: a Review
American Journal of Primatology 74:696–711 (2012) REVIEW ARTICLE Agroecosystems and Primate Conservation in The Tropics: A Review ∗ ALEJANDRO ESTRADA1 , BECKY E. RABOY2,3, AND LEONARDO C. OLIVEIRA3-6 1Estaci´on de Biolog´ıa Tropical Los Tuxtlas Instituto de Biolog´ıa, Universidad Nacional Aut´onoma de M´exico, Mexico City, Mexico 2Conservation Ecology Center, Smithsonian Conservation Biology Institute, National Zoological Park, Washington, DC 3Instituto de Estudos S´ocioambientais do Sul da Bahia (IESB), Ilh´eus-BA, Brazil 4Programa de P´os-Gradua¸c˜ao em Ecologia, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil 5Programa de P´os-Gradua¸c˜ao em Ecologia e Conserva¸c˜ao da Biodiversidade, Universidade Estadual de Santa Cruz, Ilh´eus-BA, Brazil 6Bicho do Mato Instituto de Pesquisa, Belo Horizonte-MG, Brazil Agroecosystems cover more than one quarter of the global land area (ca. 50 million km2) as highly simplified (e.g. pasturelands) or more complex systems (e.g. polycultures and agroforestry systems) with the capacity to support higher biodiversity. Increasingly more information has been published about primates in agroecosystems but a general synthesis of the diversity of agroecosystems that primates use or which primate taxa are able to persist in these anthropogenic components of the landscapes is still lacking. Because of the continued extensive transformation of primate habitat into human-modified landscapes, it is important to explore the extent to which agroecosystems are used by primates. In this article, we reviewed published information on the use of agroecosystems by primates in habitat countries and also discuss the potential costs and benefits to human and nonhuman primates of primate use of agroecosystems. -

Dhamma Vanam: Trees of Enlightenment of Buddhas
© 2021 JETIR June 2021, Volume 8, Issue 6 www.jetir.org (ISSN-2349-5162) Dhamma Vanam: Trees of Enlightenment of Buddhas 1 Dr.Shaik Ameer Jani*, 2 Ven. Andhra Analayo 1 Research Associate, 2 Founder Chairman 1 T 4-3-2 Mudfort, Secunderabad, Telangana State, India* 2 15-87/13/1, Bouddha Dhammapitamu Trust, Undrajavaram, West Godavari District, Andhra Pradesh State, India Abstract*: Plants have memory and a very well organized sensing system. They are aware of the space surrounding them. They are capable of self and non-self recognition and can take defined actions to mitigate and control diverse environmental stimuli. Ancient sages, who lived in an environment of trees and mountains perceived this truth of plants, precisely choose a few among them for their meditation, enlightenment and also actively promoted conservation of them. N,N-DMT is a psychoactive drug related with mysticism is present in both plants and humans. The study of plants associated with faith and tradition is known as Divine Botany. Lord Buddha was under Saraca asoca (Roxb.) De Wilde, Ficus religiosa (L.) and Shorea robusta (Gaertn.f.) during birth, enlightenment and liberation respectively. ‘Kshudraka Nikaya’ is a collection of minor Buddhist discourses which contains a book by name Buddhavamsha. It was translated into English as ‘The Great Chronicle of Buddhas’ by Ven. Bhadanta Vicittasarabhivamsa in 1992. Trees of enlightenment were termed as Wisdom trees. ‘The Great Chronicle of Buddhas’ listed wisdom trees of 25 past Buddha’s and future Maitreya Buddha. Wisdom trees of Tanhankara Buddha, Medhankara Buddha and Sharanamkara Buddha Bhagavan were not quoted in Buddhavamsha. -

Colobus Angolensis Palliatus) in an East African Coastal Forest
African Primates 7 (2): 203-210 (2012) Activity Budgets of Peters’ Angola Black-and-White Colobus (Colobus angolensis palliatus) in an East African Coastal Forest Zeno Wijtten1, Emma Hankinson1, Timothy Pellissier2, Matthew Nuttall1 & Richard Lemarkat3 1Global Vision International (GVI) Kenya 2University of Winnipeg, Winnipeg, Canada 3Kenyan Wildlife Services (KWS), Kenya Abstract: Activity budgets of primates are commonly associated with strategies of energy conservation and are affected by a range of variables. In order to establish a solid basis for studies of colobine monkey food preference, food availability, group size, competition and movement, and also to aid conservation efforts, we studied activity of individuals from social groups of Colobus angolensis palliatus in a coastal forest patch in southeastern Kenya. Our observations (N = 461 hours) were conducted year-round, over a period of three years. In our Colobus angolensis palliatus study population, there was a relatively low mean group size of 5.6 ± SD 2.7. Resting, feeding, moving and socializing took up 64%, 22%, 3% and 4% of their time, respectively. In the dry season, as opposed to the wet season, the colobus increased the time they spent feeding, traveling, and being alert, and decreased the time they spent resting. General activity levels and group sizes are low compared to those for other populations of Colobus spp. We suggest that Peters’ Angola black-and-white colobus often live (including at our study site) under low preferred-food availability conditions and, as a result, are adapted to lower activity levels. With East African coastal forest declining rapidly, comparative studies focusing on C. -

World's Most Endangered Primates
Primates in Peril The World’s 25 Most Endangered Primates 2016–2018 Edited by Christoph Schwitzer, Russell A. Mittermeier, Anthony B. Rylands, Federica Chiozza, Elizabeth A. Williamson, Elizabeth J. Macfie, Janette Wallis and Alison Cotton Illustrations by Stephen D. Nash IUCN SSC Primate Specialist Group (PSG) International Primatological Society (IPS) Conservation International (CI) Bristol Zoological Society (BZS) Published by: IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), Bristol Zoological Society (BZS) Copyright: ©2017 Conservation International All rights reserved. No part of this report may be reproduced in any form or by any means without permission in writing from the publisher. Inquiries to the publisher should be directed to the following address: Russell A. Mittermeier, Chair, IUCN SSC Primate Specialist Group, Conservation International, 2011 Crystal Drive, Suite 500, Arlington, VA 22202, USA. Citation (report): Schwitzer, C., Mittermeier, R.A., Rylands, A.B., Chiozza, F., Williamson, E.A., Macfie, E.J., Wallis, J. and Cotton, A. (eds.). 2017. Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA. 99 pp. Citation (species): Salmona, J., Patel, E.R., Chikhi, L. and Banks, M.A. 2017. Propithecus perrieri (Lavauden, 1931). In: C. Schwitzer, R.A. Mittermeier, A.B. Rylands, F. Chiozza, E.A. Williamson, E.J. Macfie, J. Wallis and A. Cotton (eds.), Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018, pp. 40-43. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA.