A Gas Dynamics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lurking in the Shadows: Wide-Separation Gas Giants As Tracers of Planet Formation
Lurking in the Shadows: Wide-Separation Gas Giants as Tracers of Planet Formation Thesis by Marta Levesque Bryan In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy CALIFORNIA INSTITUTE OF TECHNOLOGY Pasadena, California 2018 Defended May 1, 2018 ii © 2018 Marta Levesque Bryan ORCID: [0000-0002-6076-5967] All rights reserved iii ACKNOWLEDGEMENTS First and foremost I would like to thank Heather Knutson, who I had the great privilege of working with as my thesis advisor. Her encouragement, guidance, and perspective helped me navigate many a challenging problem, and my conversations with her were a consistent source of positivity and learning throughout my time at Caltech. I leave graduate school a better scientist and person for having her as a role model. Heather fostered a wonderfully positive and supportive environment for her students, giving us the space to explore and grow - I could not have asked for a better advisor or research experience. I would also like to thank Konstantin Batygin for enthusiastic and illuminating discussions that always left me more excited to explore the result at hand. Thank you as well to Dimitri Mawet for providing both expertise and contagious optimism for some of my latest direct imaging endeavors. Thank you to the rest of my thesis committee, namely Geoff Blake, Evan Kirby, and Chuck Steidel for their support, helpful conversations, and insightful questions. I am grateful to have had the opportunity to collaborate with Brendan Bowler. His talk at Caltech my second year of graduate school introduced me to an unexpected population of massive wide-separation planetary-mass companions, and lead to a long-running collaboration from which several of my thesis projects were born. -

Hubbl E Space T El Escope Wfpc2 Imaging of Fs Tauri and Haro 6-5B1 John E.Krist,2 Karl R.Stapelfeldt,3 Christopher J.Burrows,2,4 Gilda E.Ballester,5 John T
THE ASTROPHYSICAL JOURNAL, 501:841È852, 1998 July 10 ( 1998. The American Astronomical Society. All rights reserved. Printed in U.S.A. HUBBL E SPACE T EL ESCOPE WFPC2 IMAGING OF FS TAURI AND HARO 6-5B1 JOHN E.KRIST,2 KARL R.STAPELFELDT,3 CHRISTOPHER J.BURROWS,2,4 GILDA E.BALLESTER,5 JOHN T. CLARKE,5 DAVID CRISP,3 ROBIN W.EVANS,3 JOHN S.GALLAGHER III,6 RICHARD E.GRIFFITHS,7 J. JEFF HESTER,8 JOHN G.HOESSEL,6 JON A.HOLTZMAN,9 JEREMY R.MOULD,10 PAUL A. SCOWEN,8 JOHN T.TRAUGER,3 ALAN M. WATSON,11 AND JAMES A. WESTPHAL12 Received 1997 December 18; accepted 1998 February 16 ABSTRACT We have observed the Ðeld of FS Tauri (Haro 6-5) with the Wide Field Planetary Camera 2 on the Hubble Space Telescope. Centered on Haro 6-5B and adjacent to the nebulous binary system of FS Tauri A there is an extended complex of reÑection nebulosity that includes a di†use, hourglass-shaped structure. H6-5B, the source of a bipolar jet, is not directly visible but appears to illuminate a compact, bipolar nebula which we assume to be a protostellar disk similar to HH 30. The bipolar jet appears twisted, which explains the unusually broad width measured in ground-based images. We present the Ðrst resolved photometry of the FS Tau A components at visual wavelengths. The Ñuxes of the fainter, eastern component are well matched by a 3360 K blackbody with an extinction ofAV \ 8. For the western star, however, any reasonable, reddened blackbody energy distribution underestimates the K-band photometry by over 2 mag. -

Exep Science Plan Appendix (SPA) (This Document)
ExEP Science Plan, Rev A JPL D: 1735632 Release Date: February 15, 2019 Page 1 of 61 Created By: David A. Breda Date Program TDEM System Engineer Exoplanet Exploration Program NASA/Jet Propulsion Laboratory California Institute of Technology Dr. Nick Siegler Date Program Chief Technologist Exoplanet Exploration Program NASA/Jet Propulsion Laboratory California Institute of Technology Concurred By: Dr. Gary Blackwood Date Program Manager Exoplanet Exploration Program NASA/Jet Propulsion Laboratory California Institute of Technology EXOPDr.LANET Douglas Hudgins E XPLORATION PROGRAMDate Program Scientist Exoplanet Exploration Program ScienceScience Plan Mission DirectorateAppendix NASA Headquarters Karl Stapelfeldt, Program Chief Scientist Eric Mamajek, Deputy Program Chief Scientist Exoplanet Exploration Program JPL CL#19-0790 JPL Document No: 1735632 ExEP Science Plan, Rev A JPL D: 1735632 Release Date: February 15, 2019 Page 2 of 61 Approved by: Dr. Gary Blackwood Date Program Manager, Exoplanet Exploration Program Office NASA/Jet Propulsion Laboratory Dr. Douglas Hudgins Date Program Scientist Exoplanet Exploration Program Science Mission Directorate NASA Headquarters Created by: Dr. Karl Stapelfeldt Chief Program Scientist Exoplanet Exploration Program Office NASA/Jet Propulsion Laboratory California Institute of Technology Dr. Eric Mamajek Deputy Program Chief Scientist Exoplanet Exploration Program Office NASA/Jet Propulsion Laboratory California Institute of Technology This research was carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration. © 2018 California Institute of Technology. Government sponsorship acknowledged. Exoplanet Exploration Program JPL CL#19-0790 ExEP Science Plan, Rev A JPL D: 1735632 Release Date: February 15, 2019 Page 3 of 61 Table of Contents 1. -

The Birth of Stars and Planets
Unit 6: The Birth of Stars and Planets This material was developed by the Friends of the Dominion Astrophysical Observatory with the assistance of a Natural Science and Engineering Research Council PromoScience grant and the NRC. It is a part of a larger project to present grade-appropriate material that matches 2020 curriculum requirements to help students understand planets, with a focus on exoplanets. This material is aimed at BC Grade 6 students. French versions are available. Instructions for teachers ● For questions and to give feedback contact: Calvin Schmidt [email protected], ● All units build towards the Big Idea in the curriculum showing our solar system in the context of the Milky Way and the Universe, and provide background for understanding exoplanets. ● Look for Ideas for extending this section, Resources, and Review and discussion questions at the end of each topic in this Unit. These should give more background on each subject and spark further classroom ideas. We would be happy to help you expand on each topic and develop your own ideas for your students. Contact us at the [email protected]. Instructions for students ● If there are parts of this unit that you find confusing, please contact us at [email protected] for help. ● We recommend you do a few sections at a time. We have provided links to learn more about each topic. ● You don’t have to do the sections in order, but we recommend that. Do sections you find interesting first and come back and do more at another time. ● It is helpful to try the activities rather than just read them. -

Abstracts Connecting to the Boston University Network
20th Cambridge Workshop: Cool Stars, Stellar Systems, and the Sun July 29 - Aug 3, 2018 Boston / Cambridge, USA Abstracts Connecting to the Boston University Network 1. Select network ”BU Guest (unencrypted)” 2. Once connected, open a web browser and try to navigate to a website. You should be redirected to https://safeconnect.bu.edu:9443 for registration. If the page does not automatically redirect, go to bu.edu to be brought to the login page. 3. Enter the login information: Guest Username: CoolStars20 Password: CoolStars20 Click to accept the conditions then log in. ii Foreword Our story starts on January 31, 1980 when a small group of about 50 astronomers came to- gether, organized by Andrea Dupree, to discuss the results from the new high-energy satel- lites IUE and Einstein. Called “Cool Stars, Stellar Systems, and the Sun,” the meeting empha- sized the solar stellar connection and focused discussion on “several topics … in which the similarity is manifest: the structures of chromospheres and coronae, stellar activity, and the phenomena of mass loss,” according to the preface of the resulting, “Special Report of the Smithsonian Astrophysical Observatory.” We could easily have chosen the same topics for this meeting. Over the summer of 1980, the group met again in Bonas, France and then back in Cambridge in 1981. Nearly 40 years on, I am comfortable saying these workshops have evolved to be the premier conference series for cool star research. Cool Stars has been held largely biennially, alternating between North America and Europe. Over that time, the field of stellar astro- physics has been upended several times, first by results from Hubble, then ROSAT, then Keck and other large aperture ground-based adaptive optics telescopes. -

Sirius Astronomer
September 2015 Free to members, subscriptions $12 for 12 issues Volume 42, Number 9 Jeff Horne created this image of the crater Copernicus on September 13, 2005 from his observing site in Irvine. September 19 is International Observe The Moon Night, so get out there and have a look at a source of light pollution we really don’t mind! OCA MEETING STAR PARTIES COMING UP The free and open club meeng will The Black Star Canyon site will open on The next session of the Beginners be held September 18 at 7:30 PM in September 5. The Anza site will be open on Class will be held at the Heritage Mu‐ the Irvine Lecture Hall of the Hashing‐ September 12. Members are encouraged to seum of Orange County at 3101 West er Science Center at Chapman Univer‐ check the website calendar for the latest Harvard Street in Santa Ana on Sep‐ sity in Orange. This month, JPL’s Dr. updates on star pares and other events. tember 4. The following class will be Dave Doody will discuss the Grand held October 2. Finale of the historic Cassini mission to Please check the website calendar for the Saturn in 2017! outreach events this month! Volunteers are GOTO SIG: TBA always welcome! Astro‐Imagers SIG: Sept. 8, Oct. 13 NEXT MEETINGS: October 9, Novem‐ Remote Telescopes: TBA You are also reminded to check the web ber 13 Astrophysics SIG: Sept. 11, Oct. 16 site frequently for updates to the calendar Dark Sky Group: TBA of events and other club news. -
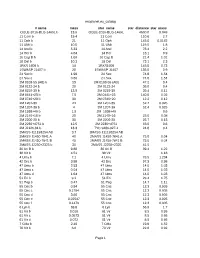
Exoplanet.Eu Catalog Page 1 # Name Mass Star Name
exoplanet.eu_catalog # name mass star_name star_distance star_mass OGLE-2016-BLG-1469L b 13.6 OGLE-2016-BLG-1469L 4500.0 0.048 11 Com b 19.4 11 Com 110.6 2.7 11 Oph b 21 11 Oph 145.0 0.0162 11 UMi b 10.5 11 UMi 119.5 1.8 14 And b 5.33 14 And 76.4 2.2 14 Her b 4.64 14 Her 18.1 0.9 16 Cyg B b 1.68 16 Cyg B 21.4 1.01 18 Del b 10.3 18 Del 73.1 2.3 1RXS 1609 b 14 1RXS1609 145.0 0.73 1SWASP J1407 b 20 1SWASP J1407 133.0 0.9 24 Sex b 1.99 24 Sex 74.8 1.54 24 Sex c 0.86 24 Sex 74.8 1.54 2M 0103-55 (AB) b 13 2M 0103-55 (AB) 47.2 0.4 2M 0122-24 b 20 2M 0122-24 36.0 0.4 2M 0219-39 b 13.9 2M 0219-39 39.4 0.11 2M 0441+23 b 7.5 2M 0441+23 140.0 0.02 2M 0746+20 b 30 2M 0746+20 12.2 0.12 2M 1207-39 24 2M 1207-39 52.4 0.025 2M 1207-39 b 4 2M 1207-39 52.4 0.025 2M 1938+46 b 1.9 2M 1938+46 0.6 2M 2140+16 b 20 2M 2140+16 25.0 0.08 2M 2206-20 b 30 2M 2206-20 26.7 0.13 2M 2236+4751 b 12.5 2M 2236+4751 63.0 0.6 2M J2126-81 b 13.3 TYC 9486-927-1 24.8 0.4 2MASS J11193254 AB 3.7 2MASS J11193254 AB 2MASS J1450-7841 A 40 2MASS J1450-7841 A 75.0 0.04 2MASS J1450-7841 B 40 2MASS J1450-7841 B 75.0 0.04 2MASS J2250+2325 b 30 2MASS J2250+2325 41.5 30 Ari B b 9.88 30 Ari B 39.4 1.22 38 Vir b 4.51 38 Vir 1.18 4 Uma b 7.1 4 Uma 78.5 1.234 42 Dra b 3.88 42 Dra 97.3 0.98 47 Uma b 2.53 47 Uma 14.0 1.03 47 Uma c 0.54 47 Uma 14.0 1.03 47 Uma d 1.64 47 Uma 14.0 1.03 51 Eri b 9.1 51 Eri 29.4 1.75 51 Peg b 0.47 51 Peg 14.7 1.11 55 Cnc b 0.84 55 Cnc 12.3 0.905 55 Cnc c 0.1784 55 Cnc 12.3 0.905 55 Cnc d 3.86 55 Cnc 12.3 0.905 55 Cnc e 0.02547 55 Cnc 12.3 0.905 55 Cnc f 0.1479 55 -

Astronomy 2015 Sample Test.Pdf
Science Olympiad Astronomy C Division Event Sample Exam Stellar Evolution: Star and Planet Formation 2014-2015 Team Number: Team Name: Instructions: 1) Please turn in all materials at the end of the event. 2) Do not forget to put your team name and team number at the top of all answer pages. 3) Write all answers on the answer pages. Any marks elsewhere will not be scored. 4) All quantitative answers are expected to have a precision of 3 or more significant figures. 5) Please do not access the internet during the event. If you do so, your team will be disqualified. 6) This test was downloaded from: www.aavso.org/science-olympiad-2015. 7) Good luck! And may the stars be with you! 1 Section A: Use Image/Illustration Set A to answer Questions 1-19. This section focuses on qualitative understanding of stellar evolution, specifically relating to star formation and planets. 1. A schematic of a T-Tauri star is shown in Image A1. (a) Which point (A-F) marks the location of the disk surrounding the protostar? (b) Which point (A-F) displays the bipolar outflow that may form Herbig-Haro objects? (c) Which point (A-F) shows the strongly variable hot spots on the protostar? 2. A color-magnitude diagram for a sample of brown dwarfs is shown in Image A2. The x-axis shows the J-K color index, while the y-axis displays J-band magnitude. The different colors represent different brown dwarf spectral types. (a) Which lettered region (A-D) corresponds approximately to a spectral type L2 brown dwarf? (b) Which lettered region (A-D) corresponds approximately to a spectral type T6 brown dwarf? (c) Which lettered region (A-D) corresponds approximately to the brown dwarf L-T type transition? 3. -

Spectral and Atmospheric Characterization of 51 Eridani B Using VLT/SPHERE?,?? M
A&A 603, A57 (2017) Astronomy DOI: 10.1051/0004-6361/201629767 & c ESO 2017 Astrophysics Spectral and atmospheric characterization of 51 Eridani b using VLT/SPHERE?,?? M. Samland1; 2, P. Mollière1; 2, M. Bonnefoy3, A.-L. Maire1, F. Cantalloube1; 3; 4, A. C. Cheetham5, D. Mesa6, R. Gratton6, B. A. Biller7; 1, Z. Wahhaj8, J. Bouwman1, W. Brandner1, D. Melnick9, J. Carson9; 1, M. Janson10; 1, T. Henning1, D. Homeier11, C. Mordasini12, M. Langlois13; 14, S. P. Quanz15, R. van Boekel1, A. Zurlo16; 17; 14, J. E. Schlieder18; 1, H. Avenhaus16; 17; 15, J.-L. Beuzit3, A. Boccaletti19, M. Bonavita7; 6, G. Chauvin3, R. Claudi6, M. Cudel3, S. Desidera6, M. Feldt1, T. Fusco4, R. Galicher19, T. G. Kopytova1; 2; 20; 21, A.-M. Lagrange3, H. Le Coroller14, P. Martinez22, O. Moeller-Nilsson1, D. Mouillet3, L. M. Mugnier4, C. Perrot19, A. Sevin19, E. Sissa6, A. Vigan14, and L. Weber5 (Affiliations can be found after the references) Received 21 September 2016 / Accepted 10 April 2017 ABSTRACT Context. 51 Eridani b is an exoplanet around a young (20 Myr) nearby (29.4 pc) F0-type star, which was recently discovered by direct imaging. It is one of the closest direct imaging planets in angular and physical separation (∼0.500, ∼13 au) and is well suited for spectroscopic analysis using integral field spectrographs. Aims. We aim to refine the atmospheric properties of the known giant planet and to constrain the architecture of the system further by searching for additional companions. Methods. We used the extreme adaptive optics instrument SPHERE at the Very Large Telescope (VLT) to obtain simultaneous dual-band imaging with IRDIS and integral field spectra with IFS, extending the spectral coverage of the planet to the complete Y- to H-band range and providing ad- ditional photometry in the K12-bands (2.11, 2:25 µm). -

LIST of PUBLICATIONS Aryabhatta Research Institute of Observational Sciences ARIES (An Autonomous Scientific Research Institute
LIST OF PUBLICATIONS Aryabhatta Research Institute of Observational Sciences ARIES (An Autonomous Scientific Research Institute of Department of Science and Technology, Govt. of India) Manora Peak, Naini Tal - 263 129, India (1955−2020) ABBREVIATIONS AA: Astronomy and Astrophysics AASS: Astronomy and Astrophysics Supplement Series ACTA: Acta Astronomica AJ: Astronomical Journal ANG: Annals de Geophysique Ap. J.: Astrophysical Journal ASP: Astronomical Society of Pacific ASR: Advances in Space Research ASS: Astrophysics and Space Science AE: Atmospheric Environment ASL: Atmospheric Science Letters BA: Baltic Astronomy BAC: Bulletin Astronomical Institute of Czechoslovakia BASI: Bulletin of the Astronomical Society of India BIVS: Bulletin of the Indian Vacuum Society BNIS: Bulletin of National Institute of Sciences CJAA: Chinese Journal of Astronomy and Astrophysics CS: Current Science EPS: Earth Planets Space GRL : Geophysical Research Letters IAU: International Astronomical Union IBVS: Information Bulletin on Variable Stars IJHS: Indian Journal of History of Science IJPAP: Indian Journal of Pure and Applied Physics IJRSP: Indian Journal of Radio and Space Physics INSA: Indian National Science Academy JAA: Journal of Astrophysics and Astronomy JAMC: Journal of Applied Meterology and Climatology JATP: Journal of Atmospheric and Terrestrial Physics JBAA: Journal of British Astronomical Association JCAP: Journal of Cosmology and Astroparticle Physics JESS : Jr. of Earth System Science JGR : Journal of Geophysical Research JIGR: Journal of Indian -

The Electrical Experimenter 529
Ksectio:),_ Lqui7en AN ELECTRO - GYRO - CRUISER. SEE PAGE 54 2 www.americanradiohistory.com ADE (ISOLATORS 1,00010 I;000,0110 VOLTS Employed -by U. S. NAVY r MARK. and all the Commercial Wireless RED. U.S. PAT. OFF. & FOREIGN COUNTRIES. Telegraph Companies ' INSULATION LOUIS STEINBERGER'S PATENTS rtai© ©401116t "' 4500 -1.509 450S 4517 u.%aóiá/iij 6350 <4ij%'.7/j 6977 A ss 68 :13 7370 6..52 SOLE MANUFACTURERS 66-76 Front St. BROOKLYN, N. Y. 60 -72 Washington St. AMERICA NEW PRICES Effective January 15, 1916. All previous prices void. _01111111111111111111111111111 1111 II111111111111111111111111111111_` 13' s' Cut shows Ih, The Brandes Switch Contact nts "Superior " Type Trans - Atlantic Brandes Head Type Receivers We are in a position to furnish Set. Price, com- (price $9) are prompt shipment of the follow- plete with ger- Used in the = ing sizes of switch points: luan silver head- Eiffel Tower to take 6 -32 screw. band, $5. Station, Paris. Tapped No. Diam. Height Doz. 50 100 626..34 inch 0 inch $ 30 $1.00 $1.75 "Astonished at its Value for the Money" 628..% inch g inch .30 .90 1.50 With 34 inch shank threaded 6 -32 627..34 inch / inch $.36 $1.25 $2.00 THAT'S what hundreds of purchasers of our "Supe- Nickel points 50 per cent. advance. rior" Receivers have said. Postage extra. "The best receiver sold at $5 to -day" is what dealers tell us. The value comes in the perfectly matched tone; in the The "Albany "Combination Detector strong, rigid -yet light- construction; in the unusually handsome finish. -

WIS-2015-07-Radioastronomie ALMA Teil4.Pdf (Application/Pdf 4.0
Das Projekt ALMA Mater* Teil 4: Eine Beobachtung, die es in sich hat: eine „Kinderstube“ für Planeten *Wir verwenden die Bezeichnung Alma Mater als Synonym für eine Universität. Seinen Ursprung hat das Doppelwort im Lateinischen (alma: nähren, mater: Mutter). Im übertragenen Sinne ernährt die (mütterliche) Universität ihre Studenten mit Wissen. Und weiter gesponnen ernährt das Projekt ALMA auch die Schüler und Studenten mit Anreizen für das Lernen. (Zudem bedeutet das spanische Wort ‚Alma‘: Seele.) In Bezug (Materie bei T-Tauri-Sternen) zum Beitrag „Der Staubring von GG Tauri“ von Wolfgang Brandner in der Zeitschrift „Sterne und Weltraum“ (SuW) 7/2015, S.30/31, WIS-ID: 1285836 Olaf Fischer Im folgenden WIS-Beitrag steht ein atemberaubendes Beobachtungsergebnis von ALMA im Brennpunkt – die detaillierte Abbildung einer protoplanetaren Scheibe um einen entstehenden Stern – die potentielle Geburtsstätte für Planeten. Neben Beschreibungen und Erklärungen werden vor allem verschiedenartige Aktivitäten (Rechnungen zur Ma und Ph, Arbeit mit Karten, Bildauswertung, Diagramminterpretation, Papiermodell, Quartett) für Schüler angeboten, um diese Beobachtung und damit im Zusammenhang stehende Inhalte (insbesondere die Sternentstehung) besser zu verstehen, auch indem diese den Nutzen des Schulwissen entdecken. Der Wert von Kenntnissen auf verschiedenen Gebieten (Sprache, Mathematik, Naturwis- senschaft, Technik) wird spürbar. Der Beitrag eignet sich als Grundlage für Schülervorträge, die Arbeit in einer AG, wie auch für den Fachunterricht in der Oberstufe.