Aflatoxin Occurrence in Nuts Consumed in Arak, Iran
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Resumes-RS-04 AB
Awards and Achievements • Winner of the Farabi International Festival (on Humanities And Islamic Studies), Third prize in education and psychology division (2012) • Outstanding Researcher at Tarbiat Modarres University (2012) • Winner of National Outstanding Thesis/Doctoral Dissertation Advisor Award by Academic Center for Education, Culture and Research (ACEVR) /Iran (2010) • Translator of the Worthy of Appreciation book on 8th National Quarterly Best Book award and 7th Annual Islamic Republic of Iran Best Book Award for the: Forms of Curriculum Inquiry (2009) • Outstanding Faculty Award at the Humanities Department of Tarbiat Modarres University (2009) • Outstanding Faculty Award, Ministry of Science, Research and Technology/Iran (2008) • Outstanding Faculty Award at Tarbiat Modarres University/Iran (2004) • Outstanding dissertation advisor award, 5th National Ferdowsi Festival, Ferdowsi University, Mashhad, Iran (2002) Publications I. Books 1. Mehrmohammadi, M.; Kian, M. (2018). Art Curriculum and Teaching in Education. Tehran: SAMT Publishing House. 2. Mehrmohammadi, M. et al. (2014). Curriculum: Theories, Approaches and Perspectives (3rd Edicion). Tehran: SAMT Publishing House. 3. Joseph, P. B. (2014). Cultures of Curriculum. Translated into Farsi by Mahmoud Mehrmohammadi. Tehran: SAMT Publishing House. 4. Mehrmohammadi, M. et al. (2013). An introduction to Teaching in higher education; Towards Faculties as pedagogic researchers. Tehran: Tarbiat Modarres University Publishing. 5. Mehrmohammadi, M. (2013). Speculative Essays in Education. Tehran: Tarbiat Modarres University Publishing House. 6. Mehrmohammadi, M. (2008). Rethinking Teaching – Learning Process and Teacher Education, with Revisions. Tehran: Madrese Publishing House. 7. Mehrmohammadi, M. (Chief Editor) et al. (2008) Forms of Curriculum Inquiry (New Edition). Tehran: Translated into Farsi. SAMT Publishing House. 8. Mehrmohammadi, M. (2004). Arts Education as General Education. -

Investigation of Students' Attitudes About the Status
International Journal of Sociology and Anthropology Research Vol.3, No.5, pp.75-85, November2017 ___Published by European Centre for Research Training and Development UK (www.eajournals.org INVESTIGATION OF STUDENTS' ATTITUDES ABOUT THE STATUS OF THE PAYAME NOOR UNIVERSITY (CASE STUDY: SAGHEZ BRANCH) Mahvash Janmardi Payame Noor University Department of Sociology, Islamic Republic of Iran ABSTRACT: The Payame Noor University (PNU), by breaking the barrier of Konkoor(University entrance examination) has faced with a rising trend of the students being admitted. The ever increasing trend of the students in current situation has made significant the necessity of the scientific structure of the university. The aim of the present research was to investigate the status of the Payame Noor University, Saghez branch from the view of the students studying at the university. Also, based on a theoretical framework and literature review, some elements of teaching staff, tutorial and self –learning books , examinations, class timelines , textbooks hours, library , electronic equipment , physical space and welfare facilities are investigated . After analyzing the data and concluding results, some recommendations are resented for the scientific promotion of the university. KEYWORDS: Payame Noor, Teaching Staff, Tutorial Books, Electronic Equipment, Student, Saghez INTRODUCTION Expert and efficient human workforce is thought to be the main source o f capital in any society. Universities contribute significantly to educating committed and effectual workforce in societies .As the most important developing institutions of the expert human resources," universities play a critical role in the path of attaining sustainable development" (Araste&Sobhani,2009). In accordance with the key role of university in promoting human force, the quality of the academic education is a fundamental strategy in materializing sustained development. -

Quality Evaluation of Payam Noor University of Isfahan in the Field of Student Based on Baldrige Excellence Model
European Online Journal of Natural and Social Sciences 2013; www.european-science.com vol.2, No. 3(s), pp. 1678-1683 ISSN 1805-3602 Quality evaluation of Payam Noor University of Isfahan in the field of student based on Baldrige Excellence Model Farangis Elyasi, Maryam Bagaii Shakhespajouh Institute, Esfahan. Iran Abstract portant characteristic of higher education has been rapid expansion of higher education institutions in de- The objective of the present study was to examine veloping countries, including our country, in the last the quality performance of Payame Noor University four decades. Indeed, higher education represents a of Isfahan about student based on Baldrige Excellence significant kind of investment in human resources that Model. Among all students and the students of econ- with providing and promoting the required knowl- omy and educational sciences who had been studying edge, skills and attitudes in the human resources helps in 2012-2013 year, the numbers of 196 people were se- to the comprehensive development of country. Thus lected randomly based on stratified sampling and Co- the higher education undertake undeniable role in the chran formula, among which 66 questionnaires were development of community, especially organizations eliminated. As a result, the analysis was performed on (Baron, 2000). University and in general, higher edu- 130 (99 females and 31 males) students. The results cation is most valuable resource that any society has show that between male and female students, there is it for the development and extension and universities a significant difference about performance qualities of have earned a great reputation in term of knowledge university and male students’ opinions are more fa- and are considered the way of researcher of science vorable than females’ in this respect. -

CV: Work and Scientific A) Personal Information Name: Amir Surname
CV: work and scientific A) Personal Information Name: Amir Surname: Ahmadi Date of Birth: 1987 Place of Birth: Tehran-IRAN Workplace: official member Payam Noor University, Department of Law Affiliation: Assistant Professor, Department of law, Payame noor University, Iran, PO BOX 19395-3697, Tehran, IRAN National number: 0077776097 Phone: +989183588179 Email: [email protected] - [email protected] Qualifications: 1. Bachelor of Islamic Law- Islamic Azad University, Hamedan 2. Bachelor of law-Payame noor University 3. MA of Islamic Law-Islamic Azad University Karaj 4. MA of Private Law from Bu Ali State University of Hamadan 5. PhD of Islamic Law- Islamic Azad University, Tehran-IRAN * B) Work experience and membership: 1-Member of faculty of Law Payame Noor University iran 2- Member of the National Foundation for Young Researchers elite Islamic Azad University 3- Four-year official member of Payame Noor University Iran 4- ID teaching at the University of Applied Science 5- Director of Scientific Society University 6- Vice Chancellor for Legal Affairs and Advisor to the President of Payame Noor University 7- Member of Legal Supervisor of University Journals 8- Member of Payame Noor University Symposium 9-Referee of law journals, University of Tehran C) Papers and conferences: 2-With ten articles ISI 3-Twenty research articles 4- Seven International Conference Include: papers 1- Title: Impacts of Contraband Goods on Family and Culture Journal Name: World Applied Programming, Vol (3), Issue (11), November 2013. 540-551 2- Title: The -

Iranian Journal of Educational Sociology (Interdisciplinary Journal of Education) Available Online At: Volume 2, Number 4, December 2019
73| Presentation of the MOOC prediction model …Volume 2, Number 4, 2019 __________________________________________________________________ Iranian journal of educational Sociology (Interdisciplinary Journal of Education) Available online at: http://www.iase-idje.ir/ Volume 2, Number 4, December 2019 Presentation of the MOOC prediction model at Payam-e-Noor University based on management / scientific / professional competence components Seyed Masoumeh Nasser Sheikholeslami1, Nazila Khatib Zanjani2 1. PhD Student, Educational Sciences, distance education planning, Payam-e-Noor, Iran. 2. Assistant Professor, Department of Educational Sciences, Payame Noor University, Iran. Article history: Abstract Received date: 18 March 2019 Purpose: The purpose of the present study was to present a Review date: 01 July 2019 predictive model of MOOC at Payam-e-Noor University based on Accepted date: 12 July 2019 the components of management and professional competence. Methodology: The research method was by purpose, applied; in terms of data type, (quantitative); in terms of data collection method or nature, and the research method was non-experimental - correlation. The statistical population of this study was 900 faculty members of Payam Noor University in Tehran. Due to the Keywords: different nature of Payame Noor University, 270 people were MOOC, management, scientific / selected as the subjects using Cochran formula and simple random professional competence sampling. The research tool consisted of a researcher-made questionnaire with 37 questions related to MOOC and two standard questionnaires of 20 and 15 questions for management variables (Wiele & Boselie Questionnaire, 2002) and professional competence (Molaeinejad questionnaire, 2012). The reliability and validity of the questionnaire were confirmed by Cronbach's alpha and reliability coefficient (MOOC, management and scientific / professional competence) of 0.7. -
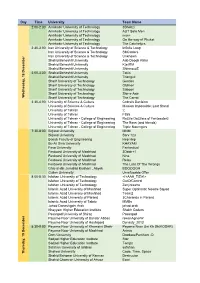
Day Time University Team Name 2:00-2:30 Amirkabir University Of
Day Time University Team Name 2:00-2:30 Amirkabir University of Technology 3Shebzi Amirkabir University of Technology AUT Safe Men Amirkabir University of Technology msn+ Amirkabir University of Technology On the way of Phuket Amirkabir University of Technology The Catchelycs 2:30-3:00 Iran University of Science & Technology Infinite Loop Iran University of Science & Technology SMCoders Iran University of Science & Technology Unknown Shahid Beheshti University Aab Doogh Khiar Shahid Beheshti University K(e)RM Shahid Beheshti University ShirmouzZ 3:00-3:30 Shahid Beheshti University Tatils Shahid Beheshti University Trianguli Sharif University of Technology Gentles Sharif University of Technology Oldmen Sharif University of Technology Saboon Sharif University of Technology Shir-e-Aab Wednesday, 18 December 18 Wednesday, Sharif University of Technology The Carrot 3:30-4:00 University of Science & Culture Cobra's Decision University of Science & Culture Mission Impossible: Last Stand University of Tehran :{ University of Tehran F355 University of Tehran - College of Engineering Razzle Dazzlers of Fantassterz University of Tehran - College of Engineering The Boss (and friends) University of Tehran - College of Engineering Triple Narengies 7:30-8:00 Birjand University MNM Birjand University Sarv 123 Bonab Faculty of Engineering Hep Hep Bu-Ali Sina University KHAYAM Fasa University Fantastical Ferdowsi University of Mashhad 3Dabir+1 Ferdowsi University of Mashhad Nine Ferdowsi University of Mashhad Relax Ferdowsi University of Mashhad The Lord -

Study of the Role of Lesson Plan on Job Performance of Secondary School Teachers in Sari City in the 2016-17
European Journal of Behavioral Sciences ISSN 2538-807X Study of The Role of Lesson Plan On Job Performance of Secondary School Teachers in Sari City in The 2016-17 Mohammad Yazdani Peraei1, Zohre Esmaeili2, Mohammad Jamalzade3 1 Degree of M.A. in Curriculum- Payame Noor University- Department of Tehran-Pardis. 2 Ph.D., Payame Noor University, faculty member. 3 Ph.D., Payame Noor University, faculty member. ARTICLE INFO ABSTRACT Keywords: the aim of this study is investigating the role of the study on job Lesson Plan performance of secondary school teachers. Job Performance for this purpose, statistical population consisted of all secondary Teacher managers of two cities of Sari (zones 1 and 2) to number 1059 in the Secondary School 2016-17 school year and questionnaires were distributed among them, including 100 male teachers and 180 female teachers, and questionnaire was analyzed by SPSS software after collecting the questionnaire. The results showed that the job performance of teachers is at a good level with an average of 75 / 34 is at a good level. and the average of the lesson was calculated on the average level (close to good). regression test was used to investigate the hypotheses of the research. based on the obtained results, the effect of knowledge use on teacher job knowledge was confirmed by a correlation coefficient of 0/546, And with the standard beta coefficient, the effect was approximately 55 %. on the other hand, the effect of the performance of the teacher 's job on teacher job performance was0/669 be and with the standard beta coefficient, the effect was 67 %. -

Dr. Abbas Teimouri Email: a [email protected], a Teimoory
CURRICULUM VITAE Name: Dr. Abbas Teimouri Email: [email protected], [email protected], [email protected], Academic position: Associate Professor of Organic chemistry Department: Payame Noor University, Isfahan, Iran Address: Kohndezh St. Chemistry department, Payame Noor University, Isfahan, Iran Tel. 0098-31-33521804-6; fax: 0098-31-33521802 Education BSc: 1995, Chemistry, Noor University of Isfahan, Isfahan, Iran MSc: 2000, Organic chemistry, Isfahan University of Technology, Isfahan, Iran PhD: 2006, Organic chemistry, Isfahan University of Technology, Isfahan, Iran THESES M.Sc.: Title of thesis: new catalysts for Fisher-Tropsh reaction, Supervisor: Prof. H.A.Dabbagh (Email: [email protected]) Ph.D.: Title of thesis: Synthesis and Reaction of sulfonyl azide dyes with aromatics, substrates, and Spectral properties, Diazotization and Diazocoupling reactions by clays, Quantum chemical Studies: Supervisor: Prof. H.A. Dabbagh (Email: [email protected]) Research Area Reactive Intermediates Asymmetric Synthesis Catalysis (Fischer-Tropsch ) Clay catalysts, Zeolite, Zirconia Reactive azo dyes Theoretical calculations in chemistry Corrosion inhibitors Tissue engineering Teaching Experience Undergraduate courses and lab demonstration for following courses Organic Chemistry I, II, III Organic Chemistry Laboratory Physical Organic Chemistry Introduction to Spectroscopy: Guide for Students of Organic Chemistry Separation and identification of organic compounds Teaching assistant for the course Organic chemistry in IUT, 1996-2005 Graduate courses 1 Advanced Organic Chemistry Heterocyclic Chemistry Organic synthesis International Conference Papers Oral presentation in: International Congress Of Chemistry And Environment INDIA at Indore (Madhya Pradesh) from 24th to 26th Dec. 2005. Pure and Applied Chemistry International Conference (PACCON 2009), NaresuanUniversity, January 14 - 16, 2009, Pitsanuloke, Thailand 16th European Symposium Organic Chemistry( ESOC 2009), Prague, Czech Republic, 12- 16 July, 2009. -

Research Note Examining the Emerging Trends in Higher
Research Note 85 Examining the Emerging Trends in Higher Education in Iran Keiko Sakurai Professor, Faculty of International Research and Education, Waseda University Director, Organization for Islamic Area Studies, Waseda University I. Introduction higher education transforms from the “elite” to the “mass,” and then to the “universal”, as the enrollment The world has witnessed new trends in higher ratio in Iran rose from under 15 percent to between education since the beginning of the 21st century. 15 and 50 percent, and then to over 50 percent2, Iran Among these, the rapid growth of tertiary enrollment, moved from the “elite” to the “mass” phase in the the increasing role of the private sector in higher mid-1990s, and then entered the “universal” phase education, and the growing influence of globalization by 2012. In 2016, it recorded a 68.8 percent growth are considered to be the most important, and they in tertiary enrollment. As shown in Figure 1, the have drawn the attention of policymakers and tertiary enrollment ratio has increased sharply during academics.1 Though the growing influence of these the last decade. This rapid growth is attributable to trends can also be observed in Iran, the Iranian the relationship between the transformation of the government’s response to these trends has been 18-year-old population and the increasing capacity influenced by Iran’s domestic politics, international of the higher education sector. Iran’s 18-year-old relations, and cultural policies. To better understand population was 1.32 million in 1996, rose to 1.83 the extent and nature of these trends in Iran, this million in 2006, and then decreased to 1.4 million in paper focuses on three dimensions of the new trends: 2011. -

An Investigation of the Factors Affecting Performance Improvement of Public Libraries Based on Customer Relationship Management (CRM) Hamid Ghazizadeh Dr
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UNL | Libraries University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Library Philosophy and Practice (e-journal) Libraries at University of Nebraska-Lincoln May 2019 An Investigation of the Factors Affecting Performance Improvement of Public Libraries based on Customer Relationship Management (CRM) Hamid Ghazizadeh Dr. Department of Library and Information science, Payame Noor University(PNU), P.O.BOX,19395-3697, Tehran, Iran Afshin Mousavi Chelak Mohaddeseh Yadollahi Mrs. Payame Noor University Ali Akbar Khasseh Payame Noor University, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/libphilprac Part of the Library and Information Science Commons Ghazizadeh, Hamid Dr.; Mousavi Chelak, Afshin; Yadollahi, Mohaddeseh Mrs.; and Khasseh, Ali Akbar, "An Investigation of the Factors Affecting Performance Improvement of Public Libraries based on Customer Relationship Management (CRM)" (2019). Library Philosophy and Practice (e-journal). 2388. https://digitalcommons.unl.edu/libphilprac/2388 An Investigation of the Factors Affecting Performance Improvement of Public Libraries based on Customer Relationship Management (CRM) Hamid Ghazizadeh Department of Knowledge and Information Science, Payame Noor University, Iran, E-mail: [email protected] Afshin Mousavi Chelak Department of Knowledge and Information Science, Payame Noor University, Iran, E-mail: [email protected] Yadollahi, Mohaddeseh M.A. in Knowledge and Information Science, Payame Noor University, Iran. Ali Akbar Khasseh Department of Knowledge and Information Science, Payame Noor University, Iran, E-mail: [email protected] Abstract Purpose: The purpose of this study was to investigate the relationship between factors affecting the performance of libraries and the components of customer relationship management (CRM). -

Hassan Sayyad Chamani [email protected]
Hassan Sayyad Chamani [email protected] PRESENT ADDRESS: 6 Elegant Bld., Orafa St., Bahonar Ave., Tehran, Iran, PC 1979955397 Tel. 0098 21 22705129 0098 9123715191 (Iran) 007904422303 (England) PERSONAL ID Married, Born on August 1970 in Zanjan, Iran. OBJECTIVE Challenging and satisfying position as an English Teacher. EDUCATION B.A. in TEFL (Teaching English as a Foreign Language), 1991-1995 Tabriz Islamic Azad University, Tabriz, Iran. Masters in TEFL (Teaching English as a Foreign Language), 2000-2003 Garmsar Islamic Azad University, Garmsar, Iran. (Thesis Topic: Observation Effects on Instructional Behaviors of Language Teachers.) Honorary Doctor Certificate in Recognition of Life-long and Dedication to Hard Work, 2016 European Foundation for Management Development, The European Union. PHD Student in TEFL (Teaching English as a Foreign Language), Payame Noor Tehran University, Dubai, United Arab Emirates. (FCE)-(CAE)-(CPE) Course, March 2011-June 2011, EC Collage, London, England CPE Exam (Certificate of Proficiency in English), June 2011, University of Cambridge ESOL Examinations, ENGLAND EXPERIENCE Junior Teacher, Sep.1991 - March 1993, Omid Language School, Tabriz, Iran. ELT Teacher, March 1993 - Nov.1995, Pardis Video English Center (Male Branch) & Goldis Video English Center (Female Branch), Tabriz, Iran. Founder, ELT Teacher, March 1993 up to Present, Pardis English Language School, Tabriz, Iran. ELT Teacher, Nov. 1995 - Dec. 1997, Soroosh Iran Institute, Tehran, Iran. ELT Senior Teacher, Dec.1997 – June 2000, Kish language Institute, Nahid Branch, Tehran, Iran. Founder, Managing Director, ELT Teacher, June 2000 up to Present, Safir Language Academy, Tehran, Iran. ESP Lecturer, Sep. 2005 – March 2006, Tehran Azad University, South Branch, Technical College, Tehran, Iran. -

Investigating the Knowledge Management Implementation in Distance Education System in Iran
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by International Institute for Science, Technology and Education (IISTE): E-Journals Information and Knowledge Management www.iiste.org ISSN 2224-5758 (Paper) ISSN 2224-896X (Online) Vol 2, No.7, 2012 Investigating the Knowledge Management Implementation in Distance Education System in Iran Mohammad Lashkary 1, Esmaeil Khodai Matin 2* , Behzad Hassannezhad Kashani 3, Kolonel Kasraei 4 1. Assistant Professor, Department of Management, Economic and Accounting, Payame Noor University, Moallem Boulevard, Moallem71, Mashhad, Iran, 91735-433 2. Young Researchers Club, Islamic Azad University, Neyshabur Branch, Neyshabur, Iran 3. Department of Business Management, Islamic Azad University, Neyshabur Branch, Neyshabur, Iran 4. Department of management, Islamic Azad University, Qeshm Branch, Qeshm, Iran *Email of the Corresponding Author: [email protected] Abstract This study intends to ascertain the amount of basic infrastructures’ readiness to implement the knowledge management system (culture and human factors, structure and processes, technical infrastructure) and rank these elements based on their importance in Payame Noor University of Mashhad. It is a survey research and the technique which has been applied is descriptive. The statistical population of research is the faculty members of Payame Noor university of Mashhad. The whole faculty members’ opinions have been studied and the required data has been assembled through questionnaires. The questions, which have been formed the questionnaire, have been designed on the basis of Hurbert Rampersad questionnaire. The findings of the research indicate that Payame Noor University of Mashhad is not ready for the application of knowledge management in different dimensions of ‘culture and human factors’, ‘information technology infrastructure’ and ‘structure and processes’.