Invasive Crayfish in a High Desert River: Implications of Concurrent Invaders and Climate Change
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Operation of Flaming Gorge Dam Final Environmental Impact Statement
Record of Decision Operation of Flaming Gorge Dam Final Environmental Impact Statement I. Summary of Action and Background The Bureau of Reclamation (Reclamation) has completed a final environmental impact statement (EIS) on the operation of Flaming Gorge Dam. The EIS describes the potential effects of modifying the operation of Flaming Gorge Dam to assist in the recovery of four endangered fish, and their critical habitat, downstream from the dam. The four endangered fish species are Colorado pikeminnow (Ptychocheilus lucius), humpback chub (Gila cypha), razorback sucker (Xyrauchen texanus), and bonytail (Gila elegans). Reclamation would implement the proposed action by modifying the operations of Flaming Gorge Dam, to the extent possible, to achieve the flows and temperatures recommended by participants of the Upper Colorado River Endangered Fish Recovery Program (Recovery Program). Reclamation’s goal is to implement the proposed action and, at the same time, maintain and continue all authorized purposes of the Colorado River Storage Project. The purpose of the proposed action is to operate Flaming Gorge Dam to protect and assist in recovery of the populations and designated critical habitat of the four endangered fishes, while maintaining all authorized purposes of the Flaming Gorge Unit of the Colorado River Storage Project (CRSP), including those related to the development of water resources in accordance with the Colorado River Compact. As the Federal agency responsible for the operation of Flaming Gorge Dam, Reclamation was the lead agency in preparing the EIS. Eight cooperating agencies also participated in preparing this EIS: the Bureau of Indian Affairs (BIA), Bureau of Land Management, National Park Service, State of Utah Department of Natural Resources, U.S. -

Big-River Monitoring on the Colorado Plateau
I NVENTORY & M ONITORING N ETWORK Big-River Monitoring on the Colorado Plateau Dustin Perkins1, Mike Scott2, Greg Auble2, Mark Wondzell3, Chris Holmquist-Johnson2, Eric Wahlig2, Helen Thomas1, and Aneth Wight1; 1Northern Colorado Plateau Network, P.O. Box 848, Bldg. 11, Arches National Park, Moab, UT 84532 2U.S. Geological Survey, Biological Resources Discipline, FORT Science Center, 2150 Centre Ave., Building C, Fort Collins, CO 80526; 3National Park Service, Water Resources Division, 1201 Oakridge Dr., Ste. 150, Fort Collins, CO 80525 Introduction and Green rivers in Canyonlands National Park. The Yampa River is the longest relatively free-flowing river Water has always been in short supply in the western reach remaining in the Colorado River basin. The U.S., making it a consistent source of conflict. In Green River is highly regulated by Flaming Gorge Dam the Colorado River drainage, an increasing human but is partially restored below its confluence with the population fuels increased demands for water from Yampa River. There have been large-scale changes the river and its tributaries. As a result, streamflow to the Green River since Flaming Gorge Dam was in virtually all of these systems has been altered by completed in 1962. reservoirs and other water-development projects. In most cases, reduced flows have significantly altered Monitoring of these rivers and their riparian peak flows and increased base flows that structure vegetation focuses on processes that affect the river floodplain vegetation, stream-channel morphology, channel, active bars, and riparian floodplains. To get and water quality (e.g., temperature, suspended a complete picture of river conditions, the NCPN sediment, nutrients). -

Itinerary: the Yampa River: 5 Days/4 Nights
Itinerary: PO Box 1324 Moab, UT 84532 (800) 332-2439 The Yampa River: (435) 259-8229 Fax (435) 259-2226 Email: [email protected] 5 Days/4 Nights www.GriffithExp.com T h r o u g h Dinosaur National Monument O v e r v i e w of The Yampa River Meeting Place Best Western Antlers 423 West Main Street Vernal, UT 84078 Meeting Time : 6 : 3 0 pm (MDT) The evening before your trip Orientation: 6 : 3 0 pm (MDT) the day BEFORE d e p a r t u r e H e r e you will learn what to expect and prepare for, receive your dry bags, sign Assumption of Risk forms, and get a chance to ask last minute q u e s t i o n s . Morning Place : Best Western Antlers 423 West Main Street Vernal, UT 84078 M o r n i n g T i m e : 7 : 0 0 a m (MDT) Return Time : Approximately 5 : 0 0 - 6 : 0 0 P M Rapid Rating: C l a s s I I I - I V (water level dependent) # of Rapids : 16 River Miles: 72 P u t i n : Deer Lodge Park Ranger Station T a k e - out : Split Mountain boat ramp Trip Length: 5 D a y s / 4 N i g h t s Raft Type(s): O a r b o a t s , Paddleboats and Inflatable Kayaks Age Limit: Minimum Age is 10 y e a r s o l d What makes this trip special? The Yampa River through the Dinosaur National Monument has it all! As the last free-flowing river in the entire Colorado River drainage, the Yampa is incredibly wild in May and June. -

Flaming Gorge Operation Plan - May 2021 Through April 2022
Flaming Gorge Operation Plan - May 2021 through April 2022 Concurrence by Kathleen Callister, Resources Management Division Manager Kent Kofford, Provo Area Office Manager Nicholas Williams, Upper Colorado Basin Power Manager Approved by Wayne Pullan, Upper Colorado Basin Regional Director U.S. Department of the Interior Bureau of Reclamation • Interior Region 7 Upper Colorado Basin • Power Office Salt Lake City, Utah May 2021 Purpose This Flaming Gorge Operation Plan (FG-Ops) fulfills the 2006 Flaming Gorge Record of Decision (ROD) requirement for May 2021 through April 2022. The FG-Ops also completes the 4-step process outlined in the Flaming Gorge Standard Operation Procedures. The Upper Colorado Basin Power Office (UCPO) operators will fulfil the operation plan and may alter from FG-Ops due to day to day conditions, although we will attempt to stay within the boundaries of the operations defined below. Listed below are proposed operation plans for four different scenarios: moderately dry, average (above median), average (below median), and moderately wet. As of the publishing of this document, the most likely scenario is the moderately dry, however actual operations will vary with hydrologic conditions. The Upper Colorado River Endangered Fish Recovery Program (Recovery Program), the Flaming Gorge Technical Working Group (FGTWG), Flaming Gorge Working Group (FG WG), United States Fish and Wildlife Service (FWS) and Western Area Power Administration (WAPA) provided input that was considered in the development of this report. The FG-Ops describes the current hydrologic classification of the Green River Basin and the hydrologic conditions in the Yampa River Basin. The FG-Ops identifies the most likely Reach 2 peak flow magnitude and duration that is to be targeted for the upcoming spring flows. -
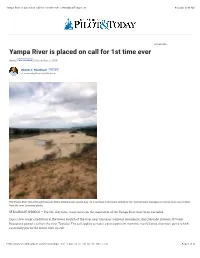
Yampa River Is Placed on Call for 1St Time Ever | Steamboattoday.Com 8/12/20, 9�43 AM
Yampa River is placed on call for 1st time ever | SteamboatToday.com 8/12/20, 943 AM YOUR AD HERE » Yampa River is placed on call for 1st time ever News FOLLOW NEWS | September 5, 2018 Eleanor C. Hasenbeck FOLLOW [email protected] The Yampa River flows through Dinosaur National Monument around Aug. 18. Low flows in the lower stretch of the river led water managers to curtail some use of water from the river. (courtesy photo) STEAMBOAT SPRINGS — For the first time, water users on the main stem of the Yampa River have been curtailed. Due to low water conditions in the lower stretch of the river near Dinosaur National Monument, the Colorado Division of Water Resources placed a call on the river Tuesday. The call applies to water users upstream from the river’s lowest diversion point, which essentially places the entire river on call. https://www.steamboatpilot.com/news/yampa-river-is-placed-on-call-for-1st-time-ever/ Page 1 of 4 Yampa River is placed on call for 1st time ever | SteamboatToday.com 8/12/20, 943 AM “We are now faced again with the Yampa River being extremely low at its lower end, and we are unable to both protect the Endangered Fishes Recovery Program reservoir water and allow all water users to continue to divert water,” Erin Light, the area division engineer, wrote in an email to water users in the Yampa River Basin on Wednesday morning. The Division of Water Resources places a call on a stream when water rights owners do not receive the amount of water they have a legal right to. -

Flaming Gorge Operation Plan - May 2020 Through April 2021
Flaming Gorge Operation Plan - May 2020 through April 2021 Concurrence by Date Kathleen Callister, Resource Management Division Chief Date ___________, Provo Area Office Manager Date ___________, Upper Colorado Power Manager Approved by Date Brent Esplin, Upper Colorado Regional Director Purpose Blue for changes that will happen This Flaming Gorge Operation Plan (FG-Ops) fulfills the 2006 Flaming Gorge Record of Decision (ROD) requirement for May 2020 through April 2021. The FG-Ops also completes the 4-step process outlined in the Flaming Gorge Standard Operation Procedures. The Upper Colorado Basin Power Office (UCPO) operators will fulfil the operation plan and may alter from FG-Ops due to day to day conditions, although we will attempt to stay within the boundaries of the operations defined below. Listed below are proposed operation plans for 3 different scenarios: moderately dry, average (above / below median), and moderately wet. As of the publishing of this document, the most likely scenario is the average (below median), however actual operations will vary with hydrologic conditions. This operation plan covers Flaming Gorge operation from May 2020 through April 2021 per the Flaming Gorge Final Environmental Impact (EIS). Both the Upper Colorado River Endangered Fish Recovery Program (Recovery Program) and the Flaming Gorge Technical Working Group (FGTWG) along with input from the Flaming Gorge Working Group, United States Fish and Wildlife Service (FWS) and Western Area Power Administration (WAPA) provided input that is incorporated into the Final FG-Ops. The 2005 Final EIS specifically addresses and outlines the content of the FGTWG proposal. This outline is used for the basis of the FG-Ops. -

Classifications and Numeric Standards for Upper Colorado River Basin and North Platte River
Presented below are water quality standards that are in effect for Clean Water Act purposes. EPA is posting these standards as a convenience to users and has made a reasonable effort to assure their accuracy. Additionally, EPA has made a reasonable effort to identify parts of the standards that are not approved, disapproved, or are otherwise not in effect for Clean Water Act purposes. November 12, 2020 Regulation No. 33 - Classifications and Numeric Standards for Upper Colorado River Basin and North Platte River Effective March 12, 2020 The following provisions are in effect for Clean Water Act purposes with these few exceptions: EPA has taken no action on: • All segment-specific total phosphorus (TP) numeric standards based on the interim value for river/stream segments with a cold water aquatic life classification (0.11 mg/L TP) or a warm water aquatic life classification (0.17 mg/L TP) • All segment-specific TP numeric standards based on the interim value for lake/reservoir segments with a warm water aquatic life classification (0.083 mg/L TP) Code of Colorado Regulations Secretary of State State of Colorado DEPARTMENT OF PUBLIC HEALTH AND ENVIRONMENT Water Quality Control Commission REGULATION NO. 33 - CLASSIFICATIONS AND NUMERIC STANDARDS FOR UPPER COLORADO RIVER BASIN AND NORTH PLATTE RIVER (PLANNING REGION 12) 5 CCR 1002-33 [Editor’s Notes follow the text of the rules at the end of this CCR Document.] 33.1 AUTHORITY These regulations are promulgated pursuant to section 25-8-101 et seq. C.R.S., as amended, and in particular, 25-8-203 and 25-8-204. -

Brown Trout in the Lees Ferry Reach of the Colorado River—Evaluation of Causal Hypotheses and Potential Interventions
Prepared in cooperation with the National Park Service, U.S. Fish and Wildlife Service, Arizona Game and Fish Department, and the Western Area Power Administration Brown Trout in the Lees Ferry Reach of the Colorado River—Evaluation of Causal Hypotheses and Potential Interventions Open-File Report 2018–1069 U.S. Department of the Interior U.S. Geological Survey Cover image: Brown trout, rainbow trout, and humpback chub. Photographs by Craig Ellsworth, Morgan Ford, Amy S. Martin, and Melissa Trammell. Prepared in cooperation with the National Park Service, U.S. Fish and Wildlife Service, Arizona Game and Fish Department, and the Western Area Power Administration Brown Trout in the Lees Ferry Reach of the Colorado River—Evaluation of Causal Hypotheses and Potential Interventions By Michael C. Runge, Charles B. Yackulic, Lucas S. Bair, Theodore A. Kennedy, Richard A. Valdez, Craig Ellsworth, Jeffrey L. Kershner, R. Scott Rogers, Melissa A. Trammell, and Kirk L. Young Open-File Report 2018–1069 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior RYAN K. ZINKE, Secretary U.S. Geological Survey William H. Werkheiser, Deputy Director exercising the authority of the Director U.S. Geological Survey, Reston, Virginia: 2018 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment—visit https://www.usgs.gov/ or call 1–888–ASK–USGS (1–888–275–8747). For an overview of USGS information products, including maps, imagery, and publications, visit https://store.usgs.gov/. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. -

Response of Native Fish Fauna to Dams in the Lower Colorado River By
Response of native fish fauna to dams in the Lower Colorado River By: Mikaela Provost ([email protected]) ECL 290 Grand Canyon Course March 8, 2017 Introduction As early as 1895 explorers and naturalists came to know the Grand Canyon as a unique system unlike any other river system in North America. One of the Canyon’s most distinguishing features, its fish fauna, was described by Evermann and Rutter (1895), both employees of the US Bureau of Fisheries who traveled extensively throughout the Colorado River Basin: “Though the families and species are very few, they are unusual interest to the student of geographic distribution… over 78% of the species of fishes now known from the Colorado Basin are peculiar to… a larger percentage of species peculiar to a single river basin than is found elsewhere in North America.” The Colorado River has the lowest diversity of fish and highest endemism of river systems in the North America, likely due to the system’s isolation and the high variability of temperature and flow conditions in the mainstem and tributaries The Grand Canyon native fish fauna is comprised of eight species; six endemic to the Grand Canyon and two, Speckled Dace (Rhinichthys osculus) and Roundtail Chub (Gila robusta), are found throughout the Colorado River Basin. Humpback Chub (Gila cyhpa), Bonytail (Gila elegans), Colorado Pikeminnow (Ptychocheilus lucius), and Razorback Sucker (Xyrauchen texanus) are listed or proposed as endangered by U.S. Fish and Wildlife Service. Flannelmouth Sucker (Catostomus latipinnis) and Bluehead Sucker (Catostomus discobolus) remain relatively common throughout the Lower Basin (Minckley et al. -

THE GUNNISON RIVER BASIN a HANDBOOK for INHABITANTS from the Gunnison Basin Roundtable 2013-14
THE GUNNISON RIVER BASIN A HANDBOOK FOR INHABITANTS from the Gunnison Basin Roundtable 2013-14 hen someone says ‘water problems,’ do you tend to say, ‘Oh, that’s too complicated; I’ll leave that to the experts’? Members of the Gunnison Basin WRoundtable - citizens like you - say you can no longer afford that excuse. Colorado is launching into a multi-generational water planning process; this is a challenge with many technical aspects, but the heart of it is a ‘problem in democracy’: given the primacy of water to all life, will we help shape our own future? Those of us who love our Gunnison River Basin - the river that runs through us all - need to give this our attention. Please read on.... Photo by Luke Reschke 1 -- George Sibley, Handbook Editor People are going to continue to move to Colorado - demographers project between 3 and 5 million new people by 2050, a 60 to 100 percent increase over today’s population. They will all need water, in a state whose water resources are already stressed. So the governor this year has asked for a State Water Plan. Virtually all of the new people will move into existing urban and suburban Projected Growth areas and adjacent new developments - by River Basins and four-fifths of them are expected to <DPSDYampa-White %DVLQ Basin move to the “Front Range” metropolis Southwest Basin now stretching almost unbroken from 6RXWKZHVW %DVLQ South Platte Basin Fort Collins through the Denver region 6RXWK 3ODWWH %DVLQ Rio Grande Basin to Pueblo, along the base of the moun- 5LR *UDQGH %DVLQ tains. -

The Colorado River an Ecological Case Study in Coupled Human and Natural Systems
The Colorado River An Ecological Case Study in Coupled Human and Natural Systems edited by David L. Alles Western Washington University e-mail: [email protected] Last Updated 2013-6-2 Note: In PDF format most of the images in this web paper can be enlarged for greater detail. 1 “Ten years or a hundred years or a hundred thousand years from now, the world's supply of freshwater will remain much the same. Such an assertion cannot be made about the world's population or about mankind's capacity for devising technologies to use-and abuse-the limited water supply. Put another way, the fate of all natural bodies of water is inseparably tied to human values about the quality of life and the number of people any part of the world can properly support. Seen from this perspective, the Colorado River is a microcosm of the world's water supply. Lessons learned from its past and policies adopted for its future are of fundamental importance not only for those dependent on the river, but also for people everywhere.” Norris C. Hundley from The West Against Itself: The Colorado River-An Institutional History http://www.movingwaters.org/hundleychapter.html 2 Introduction In studying humanity’s impact on the world’s ecosystems there are few better examples than the use and misuse of the Colorado River in the American southwest. This web paper introduces the Colorado as a case study in how humans have changed their world and the lessons that can be learned from those changes. The story of the Colorado River is a study in complex environmental systems where human and natural systems are coupled in complex loops of cause and effect. -

The Yampa River Basin, Colorado and Wyoming- a Preview to Expanded Coal-Resource Development and Its Impacts on Regional Water Resources
UNITED STATES DEPARTMENT OF THE INTERIOR WYOMING GEOLOGICAL SURVEY COLORADO THE YAMPA RIVER BASIN, COLORADO AND WYOMING- A PREVIEW TO EXPANDED COAL-RESOURCE DEVELOPMENT AND ITS IMPACTS ON REGIONAL WATER RESOURCES ^'^v^bSvi'--" WATER RESOURCES INVESTIGATIONS 78-126 BIBLIOGRAPHIC DATA 1. Report No. 2. 3. Recipient's Accession No. SHEET 4. Title and Subtitle 5. Report Date THE YAMPA RIVER BASIN, COLORADO AND WYOMING A PREVIEW TO September 1979 EXPANDED COAL-RESOURCE DEVELOPMENT AND ITS IMPACTS ON 6. REGIONAL WATER RESOURCES 7. Author(s) 8. Performing Organization Rept. T. D. Steele, D. P. Bauer, D. A. Wentz, and J. W. Warner No- USGS/WRI 78-12 9. Performing Organization Name and Address 10. Project/Task/Work Unit No. U.S. Geological Survey, Water Resources Division Box 250^6, Denver Federal Center, Mail Stop *H5 11. Contract/Grant No. Lakewood, CO 80225 12. Sponsoring Organization Name and Address 13. Type of Report & Period U.S. Geological Survey, Water Resources Division Covered Box 250^6, Denver Federal Center, Mail Stop ^15 Final Lakewood, CO 80225 14. 15. Supplementary Notes 16. Abstracts Expanded coal production and conversion in the Yampa River basin, Colorado and Wyoming, may have substantial impacts on water resources, environmental amenities, and socioeconomic conditions. Preliminary results of a 3-year basin assessment by the U.S. Geological Survey are given for evaluation of surface- and ground-water resources using available data, modeling analysis of waste-load capacity of a Yampa River reach affected by municipal wastewater-treatment plant effluents, and semiquantitative descriptions of ambient air and water-quality conditions. Aspects discussed are possible constraints on proposed development due to basin compacts and laws regulating water resources, possible change; in environmental-control regulations, and policies on energy-resource leasing and land use that will influence regional economic development.