Thieme: Imaging of the Temporal Bone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Primary Care Pediatrician and the Care of Children with Cleft Lip And/Or Cleft Palate Charlotte W
CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care The Primary Care Pediatrician Charlotte W. Lewis, MD, MPH, FAAP, a Lisa S. Jacob, DDS, MS, b Christoph U. andLehmann, MD, the FAAP, FACMI, Care c SECTION ON ORALof HEALTH Children With Cleft Lip and/or Cleft Palate Orofacial clefts, specifically cleft lip and/or cleft palate (CL/P), are among the abstract most common congenital anomalies. CL/P vary in their location and severity and comprise 3 overarching groups: cleft lip (CL), cleft lip with cleft palate (CLP), and cleft palate alone (CP). CL/P may be associated with one of many syndromes that could further complicate a child’s needs. Care of patients aDivision of General Pediatrics and Hospital Medicine, Department of with CL/P spans prenatal diagnosis into adulthood. The appropriate timing Pediatrics, University of Washington School of Medicine and Seattle Children’s Hospital, Seattle, Washington; bGeorgetown Pediatric and order of specific cleft-related care are important factors for optimizing Dentistry and Orthodontics, Georgetown, Texas; and Departments of cBiomedical Informatics and Pediatrics, Vanderbilt University Medical outcomes; however, care should be individualized to meet the specific needs Center, Nashville, Tennessee of each patient and family. Children with CL/P should receive their specialty All three authors participated extensively in developing, researching, cleft-related care from a multidisciplinary cleft or craniofacial team with and writing the manuscript and revising it based on reviewers’ comments; Dr Lehmann made additional revisions after review by the sufficient patient and surgical volume to promote successful outcomes. board of directors. The primary care pediatrician at the child’s medical home has an essential This document is copyrighted and is property of the American role in making a timely diagnosis and referral; providing ongoing health Academy of Pediatrics and its Board of Directors. -

Assessment of Bone Conduction Thresholds After Surgical Treatment in Patients with Labyrinthine Fistula
Turkish Archives of Otorhinolaryngology Turk Arch Otorhinolaryngol 2018; 56(2): 89-94 89 Türk Otorinolarengoloji Arşivi Assessment of Bone Conduction Thresholds After Surgical Treatment in Patients with Labyrinthine Fistula Müzeyyen Yıldırım Baylan1 , Ümit Yılmaz1 , Zeki Akkuş2 , İsmail Topçu1 1Department of Otorhinolaryngology, Dicle University School of Medicine, Diyarbakır, Turkey Original Investigation 2Department of Biostatistics, Dicle University School of Medicine, Diyarbakır, Turkey Abstract Objective: This study aimed to analyze the bone con- years. In the post-operative period, it was possib- duction thresholds before and after surgery in chronic le to conduct audiological follow-up on 20 patients. otitis media patients with cholesteatoma who had In these follow-ups, 16 patients showed no change labyrinthine fistula and whose cholesteatoma matrix in bone conduction thresholds, two patients showed had been completely cleaned. worsening, and two showed improvement. When Methods: The study was performed between 2013 pre- and post-operative bone conduction thresholds to 2017 with 23 chronic otitis media patients who at each frequency were compared separately, no sig- had labyrinthine fistula with cholesteatoma and who were operated at the Department of Otorhinolar- nificant difference was found (p=0.937). No statis- yngology of Dicle University School of Medicine. tically significant difference was found between the Patients were assessed by anamnesis and examina- pre- and post-operative means at the four frequencies tion and when necessary, by temporal computerized (p=0.712). tomography and diffusion magnetic resonance ima- Conclusion: In this study, we found that to reduce ging. Bone conduction thresholds at frequencies of complications relating to cholesteatoma, it might be 500, 1000, 2000, and 4000 Hz were determined by audiometric examination and they were compared necessary to completely remove the matrix especially before and after surgery. -

Soonerstart Automatic Qualifying Syndromes and Conditions 001
SoonerStart Automatic Qualifying Syndromes and Conditions 001 Abetalipoproteinemia 272.5 002 Acanthocytosis (see Abetalipoproteinemia) 272.5 003 Accutane, Fetal Effects of (see Fetal Retinoid Syndrome) 760.79 004 Acidemia, 2-Oxoglutaric 276.2 005 Acidemia, Glutaric I 277.8 006 Acidemia, Isovaleric 277.8 007 Acidemia, Methylmalonic 277.8 008 Acidemia, Propionic 277.8 009 Aciduria, 3-Methylglutaconic Type II 277.8 010 Aciduria, Argininosuccinic 270.6 011 Acoustic-Cervico-Oculo Syndrome (see Cervico-Oculo-Acoustic Syndrome) 759.89 012 Acrocephalopolysyndactyly Type II 759.89 013 Acrocephalosyndactyly Type I 755.55 014 Acrodysostosis 759.89 015 Acrofacial Dysostosis, Nager Type 756.0 016 Adams-Oliver Syndrome (see Limb and Scalp Defects, Adams-Oliver Type) 759.89 017 Adrenoleukodystrophy, Neonatal (see Cerebro-Hepato-Renal Syndrome) 759.89 018 Aglossia Congenita (see Hypoglossia-Hypodactylia) 759.89 019 Albinism, Ocular (includes Autosomal Recessive Type) 759.89 020 Albinism, Oculocutaneous, Brown Type (Type IV) 759.89 021 Albinism, Oculocutaneous, Tyrosinase Negative (Type IA) 759.89 022 Albinism, Oculocutaneous, Tyrosinase Positive (Type II) 759.89 023 Albinism, Oculocutaneous, Yellow Mutant (Type IB) 759.89 024 Albinism-Black Locks-Deafness 759.89 025 Albright Hereditary Osteodystrophy (see Parathyroid Hormone Resistance) 759.89 026 Alexander Disease 759.89 027 Alopecia - Mental Retardation 759.89 028 Alpers Disease 759.89 029 Alpha 1,4 - Glucosidase Deficiency (see Glycogenosis, Type IIA) 271.0 030 Alpha-L-Fucosidase Deficiency (see Fucosidosis) -

Research Article
z Available online at http://www.journalcra.com INTERNATIONAL JOURNAL OF CURRENT RESEARCH International Journal of Current Research Vol. 10, Issue, 07, pp.71222-71228, July, 2018 ISSN: 0975-833X RESEARCH ARTICLE THE TONGUE SPEAKS A LOT OF HEALTH. 1,*Dr. Firdous Shaikh, 2Dr. Sonia Sodhi, 3Dr Zeenat Fatema Farooqui and 4Dr. Lata Kale 1PG Student, Department of Oral Medicine and Radiology, CSMSS Dental College and Hospital, Aurangabad 2Professor, Department of Oral Medicine and Radiology, CSMSS Dental College and Hospital, Aurangabad 3Fatema Farooqui, Chief Medical Officer, Sri Ram Homeopathic Clinic and Research Center, Solapur 4Professor and Head, Department of Oral Medicine and Radiology, CSMSS Dental College and Hospital, Aurangabad ARTICLE INFO ABSTRACT Article History: Multifunctional organ of the human body without a bone yet strong is the tongue. It mainly consists Received 26th April, 2018 of the functional portion of muscle mass, mucosa, fat and the specialized tissue of taste i.e. the Received in revised form papillae. Diseases may either result from internal/ systemic causes of extrinsic causes like trauma, 14th May, 2018 infection, etc. A new method for classification has been proposed in this review for diseases of Accepted 09th June, 2018 tongue. This review mainly focuses on encompassing almost each aspect that the body reflects via its th Published online 30 July, 2018 mirror in mouth, the tongue. Key Words: Tongue, Diseases of Tongue, Discoloration of Tongue, Oral health, Hairy Tongue. Copyright © 2018, Firdous Shaikh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. -
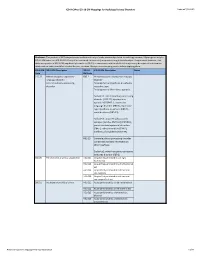
ICD-9/10 Mapping Spreadsheet
ICD-9-CM to ICD-10-CM Mappings for Audiology Related Disorders Updated 7/16/2015 Disclaimer: This product is NOT comprehensive and consists only of codes commonly related to audiology services. Mappings are only to ICD-10-CM codes, not ICD-10-PCS. Every effort was made to accurately map codes using detailed analysis. Keep in mind, however, that while many codes in ICD-9-CM map directly to codes in ICD-10, in some cases, additional clinical analysis may be required to determine which code or codes should be selected for your situation. Always review mapping results before applying them. ICD-9-CM ICD-9-CM Description ICD-10- ICD-10-CM Description Notes Code CM Code 315.32 Mixed receptive-expressive F80.2 Mixed receptive-expressive language language disorder disorder Central auditory processing Developmental dysphasia or aphasia, disorder receptive type Developmental Wernicke's aphasia Excludes1: central auditory processing disorder (H93.25), dysphasia or aphasia NOS (R47.-), expressive language disorder (F80.1), expressive type dysphasia or aphasia (F80.1), word deafness (H93.25) Excludes2: acquired aphasia with epilepsy [Landau-Kleffner] (G40.80-), pervasive developmental disorders (F84.-), selective mutism (F94.0), intellectual disabilities (F70-F79) H93.25 Central auditory processing disorder Congenital auditory imperception Word deafness Excludes1: mixed receptive-epxressive language disorder (F80.2) 380.00 Perichondritis of pinna, unspecified H61.001 Unspecified perichondritis of right external ear H61.002 Unspecified perichondritis -

Mudr. Kaliariková Seminar for Medical Students 2019/20
MUDr. Kaliariková Seminar for medical students 2019/20 Deformity of shape, cosmetic defect Therapy: plastic correction – otoplasty: Children from the age of 6 years Congenital defect of auricle development (microtia) or missing auricle (anotia) is often combined with the congenital defect of the EAC (stenosis, atresia) Auditory canal stenosis means that it is narrower than 4 mm Dg: CT, objective hearing examination (exclusion of congenital defect of the middle and inner ear or the auditory track) Conductive hearing loss HRCT of the Temporal Bone Right side – stenosis of EAC Left side – atresia of EAC It depends on examination results and on hearing affliction extent (unilateral or bilateral affliction) Aim: provide communication Hearingaid devices (BAHA) Surgery: tympanoplasty, plastic of external auditory canal or auricle External opening is placed near the tragus and the inner opening between cartilaginous and bone part of the EAC Complication – inflammation (secretion, swelling or erythema) Th: Exstirpation, ATB (inflammation) Usually connected with atresia of EAC and anomalies of auricle Isolated / part of syndroms (more common, e.g. Treacher Collins) Usually unilateral Hearing-impairment Dg: CT, objective hearing examination Th: hearing-aid devices, tympanoplasty 20% of children with SNHL (sensorineural hearing loss) have CT anomalies of inner ear Cochlear anomalies Enlarged vestibular aqueduct (EVA) Semicircular canal dysplasia Michel deformity or complete labyrinthine aplasia (cochlea + vestibulum) Cochlear aplasia Common cavity malformation to the cochlea and vestibule Cochlear hypoplasia Cochlear incomplete partition type I (including cystic cochleovestibular anomaly) Cochlear incomplete partition type II (Mondini dysplasia) 1,5 screw of cochlea, dilated aqueductus Normal cochlea 2,5-2,75 screw Dilated vestibulum Normal vestibulum Ø 60% congenital • Damage of auditory organ during development (1. -

Download Download
1 Contribution of dental private practitioners to 2 publications on anatomical variations using 3 cone beam computed tomography. 4 5 Authors: 6 Hebda A1,*MS, 7 Theys S2 DDS, 8 De Roissart J3 MD, 9 Perez E4 DDS, 10 Olszewski R1,3 DDS,MD,PhD,DrSc 11 Affiliations: 12 1 Oral and maxillofacial surgery research Lab, NMSK, IREC, SSS, UCLouvain, 13 Brussels, Belgium 14 2 Department of pediatric dentistry and special care, Cliniques universitaires saint 15 Luc, UCLouvain, Brussels, Belgium 16 3 Department of oral and maxillofacial surgery, Cliniques universitaires saint Luc, 17 UCLouvain, Brussels, Belgium 18 4 Department of orthodontics, Cliniques universitaires saint Luc, UCLouvain, 19 Brussels, Belgium 20 *Corresponding author: Hebda A, Oral and maxillofacial surgery research Lab, 21 NMSK, IREC, SSS, UCLouvain, Brussels, Belgium, ORCID Id 0000-0001-5111- 22 0021 1 2 [Nemesis] Titre de l’article (PUL - En- tête paire) 23 Disclaimer: the views expressed in the submitted article are our own and not an 24 official position of the institution or funder. 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 [Nemesis] Titre de l’article (PUL - En- tête impaire) 3 61 Abstract 62 Objective: To investigate the participation of citizens-dental private practitioner in 63 scientific articles about anatomical variations on dentomaxillofacial CBCT. Our null 64 hypothesis was that private practice practitioners are not involved in publications on 65 anatomical variations using cone beam computed tomography. -

Spectrum of Third Window Abnormalities: Semicircular Canal Dehiscence and Beyond
Published August 25, 2016 as 10.3174/ajnr.A4922 REVIEW ARTICLE Spectrum of Third Window Abnormalities: Semicircular Canal Dehiscence and Beyond X M.-L. Ho, X G. Moonis, X C.F. Halpin, and X H.D. Curtin ABSTRACT SUMMARY: Third window abnormalities are defects in the integrity of the bony structure of the inner ear, classically producing sound-/ pressure-induced vertigo (Tullio and Hennebert signs) and/or a low-frequency air-bone gap by audiometry. Specific anatomic defects include semicircular canal dehiscence, perilabyrinthine fistula, enlarged vestibular aqueduct, dehiscence of the scala vestibuli side of the cochlea, X-linked stapes gusher, and bone dyscrasias. We discuss these various entities and provide key examples from our institutional teaching file with a discussion of symptomatology, temporal bone CT, audiometry, and vestibular-evoked myogenic potentials. ABBREVIATIONS: EVAS ϭ enlarged vestibular aqueduct syndrome; SSCCD ϭ superior semicircular canal dehiscence hird window abnormalities are defects in the integrity of the ing effects decrease air conduction. The 2 physiologic windows Tbony structure of the inner ear, first described by Minor et al between the middle and inner ear are the oval window, which in 1998.1 In 2008, Merchant and Rosowski2 proposed a universal transmits vibrations from the auditory ossicles, and the round theory for the underlying mechanism of hearing loss accompany- window of the cochlea. With air conduction, there is physiologic ing these defects. Normal sound conduction is transmitted entrainment of the oval and round windows due to coupling by through the oval and round windows, which serve as fluid inter- incompressible perilymph. Pressure differences between the co- faces between air in the middle ear and perilymphatic fluid spaces chlear perilymphatic spaces activate hair cells and create the per- of the inner ear. -

A Small Road to Misery in Necrotizing External Otitis
Published August 8, 2019 as 10.3174/ajnr.A6161 ORIGINAL RESEARCH HEAD & NECK A Persistent Foramen of Huschke: A Small Road to Misery in Necrotizing External Otitis X W.L. van der Meer, X M. van Tilburg, X C. Mitea, and X A.A. Postma ABSTRACT BACKGROUND AND PURPOSE: Necrotizing external otitis is a serious complication of external otitis with different spreading patterns. A persistent foramen of Huschke is a dehiscence located antero-inferior in the osseous external ear canal and posterior-medial to the temporomandibular joint. This dehiscence can facilitate extension of infection in an anterior pattern next to classic spread along the fissures of Santorini. The aim of this study was to define the prevalence and size of a persistent foramen of Huschke in patients with necrotizing external otitis. MATERIALS AND METHODS: We retrospectively examined 78 CT temporal bone studies (39 patients with necrotizing external otitis, 39 control subjects). The side and presence of the foramen were noted, and its prevalence was calculated. The maximal width of the foramen of Huschke was measured in the axial plane and classified as subtle, mild, moderate, or extensive. RESULTS: A persistent foramen of Huschke was present in 21 patients (26 ears) and 7 control subjects (9 ears). Prevalence was 50% (20/40) and 11.5% (9/78) in affected ears of patients with necrotizing external otitis and control subjects, respectively. Almost all affected ears showed an anterior distribution pattern of necrotizing external otitis. The extensive dehiscence was most common in affected ears. CONCLUSIONS: An anterior necrotizing external otitis spreading pattern is associated with the presence and increased size of a persis- tent foramen of Huschke. -

ANATOMY of EAR Basic Ear Anatomy
ANATOMY OF EAR Basic Ear Anatomy • Expected outcomes • To understand the hearing mechanism • To be able to identify the structures of the ear Development of Ear 1. Pinna develops from 1st & 2nd Branchial arch (Hillocks of His). Starts at 6 Weeks & is complete by 20 weeks. 2. E.A.M. develops from dorsal end of 1st branchial arch starting at 6-8 weeks and is complete by 28 weeks. 3. Middle Ear development —Malleus & Incus develop between 6-8 weeks from 1st & 2nd branchial arch. Branchial arches & Development of Ear Dev. contd---- • T.M at 28 weeks from all 3 germinal layers . • Foot plate of stapes develops from otic capsule b/w 6- 8 weeks. • Inner ear develops from otic capsule starting at 5 weeks & is complete by 25 weeks. • Development of external/middle/inner ear is independent of each other. Development of ear External Ear • It consists of - Pinna and External auditory meatus. Pinna • It is made up of fibro elastic cartilage covered by skin and connected to the surrounding parts by ligaments and muscles. • Various landmarks on the pinna are helix, antihelix, lobule, tragus, concha, scaphoid fossa and triangular fossa • Pinna has two surfaces i.e. medial or cranial surface and a lateral surface . • Cymba concha lies between crus helix and crus antihelix. It is an important landmark for mastoid antrum. Anatomy of external ear • Landmarks of pinna Anatomy of external ear • Bat-Ear is the most common congenital anomaly of pinna in which antihelix has not developed and excessive conchal cartilage is present. • Corrections of Pinna defects are done at 6 years of age. -

Oral Health Abnormalities in Children Born with Macrosomia Established During Mixed Dentition Period
© Wydawnictwo Aluna Wiadomości Lekarskie 2019, tom LXXII, nr 5 cz I PRACA ORYGINALNA ORIGINAL ARTICLE ORAL HEALTH ABNORMALITIES IN CHILDREN BORN WITH MACROSOMIA ESTABLISHED DURING MIXED DENTITION PERIOD Olga V. Garmash KHARKIV NATIONAL MEDICAL UNIVERSITY, KHARKIV, UKRAINE ABSTRACT Introduction: The prevalence of soft tissue and hard tooth tissuediseases in the oral cavity and the morphofunctional disorders of craniofacial complex, require attention ofspecialistsin various branches of medicine. Scientists began to pay attention to metabolic and other violations that have occurred in the fetal development and led to the occurrence of certain changes in the dental status of the child. The aim of thisresearch is to study the features of the dental health condition in the children of Northeast of Ukraine, who were born with macrosomia during the period of mixed dentition. The study takes into account intrauterine body length growth acceleration, intrauterine obesity or well-balanced acceleration of both the body weight and length gain. Materials and methods: Thirty 6.5–11-year-old children with fetal macrosomia were examined (MainGroup). A Comparison Group was comprised of sixteen children, whose weight-height parameters at birth were normal (fetal normosomia). All children in the Main group were split into four subgroups in accordance with weight-height parameters at birth using the V. I. Grischenko and his co-authors’ harmonious coefficient. The evaluation of the hygiene status of the oral cavity, the dental caries intensity evaluation, and the quantitative analysis of minor salivary gland secretion have been performed. The prevalence of dentoalveolar abnormalities was evaluated. Results: The highest values of caries intensity were recorded in macrosomic-at-birth children born with harmonious (well-balanced) intrauterine development, with intrauterine obesity and increased body length, or with intrauterine obesity and an average body length. -

The Coexistence of Labyrinthine Fistula and the Facial Canal Dehiscence
The Mediterranean Journal of Otology ORIGINAL ARTICLE Management of Labyrinthine Fistula and Accompanying Findings: The Coexistence of Labyrinthine Fistula and the Facial Canal Dehiscence Masoud Naderpour, Ghodrat Mohammadi, Najmeh Doostmohammadian Department of Otorhinolaryngology, Tabriz University of Medical science, Tabriz, Iran OBJECTIVE: To describe the audio-vestibular results of labyrinthine fistula surgery in patients with cholesteatoma. Correspondent Author: PATIENTS AND METHODS: Data of 185 patients who had undergone Chodrat Mohammadi Dept. Otorhnotaryngology Tabriz surgery for cholesteatoma between 2001 and 2007 were reviewed. University of Medical Sciences, Three-layer sealing was used for the management of fistula. Tabriz, ‹ran RESULTS: Twenty patients were found to have labyrinthine fistula, of which 11 (55%) were male and 9(45%) female. Fistula wase located in lateral Tel: + 98- 9141141619 semicircular canal in all cases. Correlation of labyrinthine fistula and facial E-mail: [email protected] nerve dehiscence was statistically significant. Follow up was done for 1- 6 year. Postoperatively, vertigo disappeared in 19 (95 %) patients. Submitted: 14 April 2008 Revised: 10 July 2008 Hearing remained unchanged in 18 (90 %) patients. Worsening in bone Accepted: 17 July 2008 conduction thresholds was observed in 2 (10 %) patients. Postoperative deafness did not occur. Mediterr J Otol 2008; 4: 132-137 CONCLUSION: Possibility of facial nerve dehiscence and tegmen defect should be considered in patients with labyrinthine fistula. Three-layer Copyright 2005 © The Mediterranean sealing may be a valuable technique in surgical treatment of labyrinthine Society of Otology and Audiology fistula, lowering the risk of cochleovestibular functions. 132 Management of labyrinthine fistula and accompanying findings Cholesteatoma is a pocket or cystic lesion consisting of Labyrinthine fistula (LF) is encountered during stratified squamous epithelium and proliferative keratin surgery for cholesteatoma with an average frequency within the temporal bone.