A Randomized Clinical Trial Evaluating Mannitol, Lactitol, and Polyethylene Glycol Macrogol As Oral Solutions for Colonoscopy Preparation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemicals Used for Chemical Manufacturing Page 1 of 2
Chemicals used for Chemical Manufacturing Page 1 of 2 Acetic Acid (Glacial, 56%) Glycol Ether PMA Acetone Glycol Ether PNB Acrylic Acid Glycol Ether PNP Activated Carbon Glycol Ether TPM Adipic Acid Glycols Aloe Vera Grease Aluminum Stearate Gum Arabic Aluminum Sulfate Heat Transfer Fluids Amino Acid Heptane Ammonium Acetate Hexane Ammonium Bicarbonate Hydrazine Hydrate Ammonium Bifluoride Hydrochloric Acid (Muriatic) Ammonium Chloride Hydrogen Peroxide Ammonium Citrate Hydroquinone Ammonium Hydroxide Hydroxylamine Sulfate Ammonium Laureth Sulfate Ice Melter Ammonium Lauryl Sulfate Imidazole Ammonium Nitrate Isobutyl Acetate Ammonium Persulfate Isobutyl Alcohol Ammonium Silicofluoride Calcium Stearate Dipropylene Glycol Isopropanolamine Ammonium Sulfate Carboxymethylcellulose Disodium Phosphate Isopropyl Acetate Antifoams Caustic Potash D'Limonene Isopropyl Alcohol Antifreeze Caustic Soda (All Grades) Dodecylbenzene Sulfonic Acid Isopropyl Myristate Antimicrobials Caustic Soda (Beads, Prills) (DDBSA) Isopropyl Palmitate Antimony Oxide Cetyl Alcohol Dowfrost Itaconic Acid Aqua Ammonia Cetyl Palmitate Dowfrost HD Jojoba Oil Ascorbic Acid Chlorine, Granular Dowtherm SR-1 Keratin Barium Carbonate Chloroform Dowtherm 4000 Lactic Acid Barium Chloride Chromic Acid EDTA Lanolin Beeswax Citric Acid (Dry and Liquid) EDTA Plus Lauric Acid Bentonite Coal Epsom Salt Lauryl Alcohol Benzaldehyde Cocamide DEA Ethyl Acetate Lecithin Benzoic Acid Copper Nitrate Ethyl Alcohol (Denatured) Lime Benzyl Alcohol Copper Sulfate Ethylene Glycol Linoleic Acid Bicarbonate -

“Polyols: a Primer for Dietetic Professionals” Is a Self-Study
1 “Polyols: A primer for dietetic professionals” is a self-study module produced by the Calorie Control Council, an accredited provider of continuing professional education (CPE) for dietetic professionals by the Commission on Dietetic Registration. It provides one hour of level 1 CPE credit for dietetic professionals. The full text of the module is in the notes section of each page, and is accompanied by summary points and/or visuals in the box at the top of the page. Directions for obtaining CPE are provided at the end of the module. 2 After completing this module, dietetic professionals will be able to: • Define polyols. • Identify the various types of polyols found in foods. • Understand the uses and health effects of polyols in foods. • Counsel clients on how to incorporate polyols into an overall healthful eating pattern. 3 4 Polyols are carbohydrates that are hydrogenated, meaning that a hydroxyl group replaces the aldehyde or ketone group found on sugars. Hydrogenated monosaccharides include erythritol, xylitol, sorbitol, and mannitol. Hydrogenated disaccharides include lactitol, isomalt, and maltitol. And hydrogenated starch hydrolysates (HSH), or polyglycitols (a wide range of corn syrups and maltodextrins), are formed from polysaccharides (Grabitske and Slavin 2008). 5 Nearly 54 percent of Americans are trying to lose weight, more than ever before. Increasingly, they are turning toward no- and low-sugar, and reduced calorie, foods and beverages to help them achieve their weight loss goals (78% of Americans who are trying to lose weight) (CCC 2010). Polyols, found in many of these foods, are becoming a subject of more interest. 6 They are incompletely digested , therefore are sometimes referred to as “low- digestible carbohydrates.” Polyols are not calorie free, as there is some degree of digestion and absorption of the carbohydrate. -

Constipation: a Parent's Guide
CONSTIPATION: A PARENT’S GUIDE Twenty Questions About Constipation: Answers to Guide Parents and Professionals Constipation is the abnormally delayed or infrequent passage of hard stools. Most children, and many adults, too, become constipated from time to time. Often, the duration is short; occasionally it persists for months, even years. Although constipation can be uncomfortable, may create worry, and sometimes seem serious, fortunately it does not have long-term, troubling effects in most healthy children. This Booklet is designed to help you deal with childhood constipation by answering several questions and outlining management instructions for you to follow. Questions answered are: 1. What are the normal 11. What can we learn from patterns of bowel physical exam results? movements at 12. What is our treatment different ages? program for constipation? 2. What makes up bowel 13. How is the colon movements and how do cleaned out? they travel? 14. How are stools softened? 3. What is constipation? 15. Why is trying to have 4. When is constipation most bowel movements twice likely to occur? a day so important? 5. Why does constipation 16. What are the expected persist in some children? results of the treatment 6. Why would a child hold program? back stool and what 17. What do we do if the happens then? cleansing regimen is 7. How can proper toilet not successful? training help? 18. What is the long-term 8. Why would stool back up program for children in the colon? prone to constipation? 9. Why do some children 19. Does a special diet help have soiling accidents? resolve constipation? 10. -
Final Stakeholder List PDF 190 KB
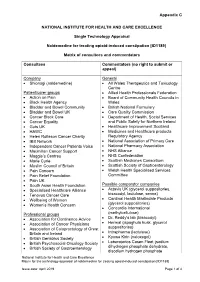
Appendix C NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Naldemedine for treating opioid-induced constipation [ID1189] Matrix of consultees and commentators Consultees Commentators (no right to submit or appeal) Company General • Shionogi (naldemedine) • All Wales Therapeutics and Toxicology Centre Patient/carer groups • Allied Health Professionals Federation • Action on Pain • Board of Community Health Councils in • Black Health Agency Wales • Bladder and Bowel Community • British National Formulary • Bladder and Bowel UK • Care Quality Commission • Cancer Black Care • Department of Health, Social Services • Cancer Equality and Public Safety for Northern Ireland • Guts UK • Healthcare Improvement Scotland • HAWC • Medicines and Healthcare products • Helen Rollason Cancer Charity Regulatory Agency • IBS Network • National Association of Primary Care • Independent Cancer Patients Voice • National Pharmacy Association • Macmillan Cancer Support • NHS Alliance • Maggie’s Centres • NHS Confederation • Marie Curie • Scottish Medicines Consortium • Muslim Council of Britain • Scottish Society of Gastroenterology • Pain Concern • Welsh Health Specialised Services • Pain Relief Foundation Committee • Pain UK • South Asian Health Foundation Possible comparator companies • Specialised Healthcare Alliance • Actavis UK (glycerol suppositories, • Tenovus Cancer Care bisacodyl, lactulose, senna) • Wellbeing of Women • Cardinal Health Martindale Products • Women’s Health Concern (glycerol suppositories) • Concordia International -

Polyethylene Glycol (PEG) 3350
Polyethylene Glycol (PEG) 3350 Polyethylene glycol is a laxative sold under the trade names MiraLAX®, ClearLax®, GaviLAX®, GlycoLax® and Purelax®. You do not need a doctor’s prescription to buy PEG 3350. It is available over-the-counter. PEG 3350 is an osmotic laxative, which means it increases the water content of stool and is effective in treating or preventing constipation. The body cannot absorb or digest this laxative. PEG 3350 is typically taken once a day by mouth, but the dose may be adjusted higher. It comes as a powder which you will have to mix with liquid. How do I prepare the medication? The standard dose is 17 grams of powder mixed into 8 ounces of liquid. The bottle has a measuring cap that is marked with a line. 1. Pour the powder into the cap up to the marked line. 2. Add the powder in the cap to a full glass (8 ounces) of water, juice, soda, coffee or tea. o If you are over 65, have kidney disease or have liver disease, please only use water. 3. Mix powder well and drink the solution. How do I take PEG 3350? You can take this medication on a full or empty stomach. PEG 3350 doesn’t have any known drug interactions but you should not take other medications at the same time that you take PEG 3350. Other medications may not be digested and absorbed as well. Always drink plenty of decaffeinated liquids with this medication. Michigan Bowel Control Program -1- What are possible side effects? Call your doctor immediately if you have any of the following side-effects: diarrhea severe bloating difficulty breathing painful swelling of the itching of the skin stomach hives vomiting skin rash Other side-effects that usually do not require immediate medical attention are: bloating cramps lower abdominal (stomach) nausea discomfort passing extra gas Some of these symptoms will decrease over time. -

Novel Insights Into Mannitol Metabolism in the Fungal Plant
Novel insights into mannitol metabolism in the fungal plant pathogen Botrytis cinerea Thierry Dulermo, Christine Rascle, Geneviève Billon-Grand, Elisabeth Gout, Richard Bligny, Pascale Cotton To cite this version: Thierry Dulermo, Christine Rascle, Geneviève Billon-Grand, Elisabeth Gout, Richard Bligny, et al.. Novel insights into mannitol metabolism in the fungal plant pathogen Botrytis cinerea. Biochemical Journal, Portland Press, 2010, 427 (2), pp.323-332. 10.1042/BJ20091813. hal-00479283 HAL Id: hal-00479283 https://hal.archives-ouvertes.fr/hal-00479283 Submitted on 30 Apr 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Biochemical Journal Immediate Publication. Published on 05 Feb 2010 as manuscript BJ20091813 1 NOVEL INSIGHTS INTO MANNITOL METABOLISM IN THE FUNGAL PLANT 2 PATHOGEN BOTRYTIS CINEREA 3 4 Authors : Thierry Dulermo*†, Christine Rascle*, Geneviève Billon-Grand*, Elisabeth Gout‡, 5 Richard Bligny‡ and Pascale Cotton§ 6 7 Address 8 *Génomique Fonctionnelle des Champignons Pathogènes des Plantes, UMR 5240 9 Microbiologie, Adaptation -

Ferring Pharmaceuticals Inc. Page 1 of 13 HIGHLIGHTS OF
HIGHLIGHTS OF PRESCRIBING INFORMATION ---------------------DOSAGE FORMS AND STRENGTHS---------------------- CLENPIQ oral solution: Each bottle contains 10 mg of sodium picosulfate, These highlights do not include all the information needed to use ® 3.5 g of magnesium oxide, and 12 g of anhydrous citric acid in 160 mL of CLENPIQ safely and effectively. See full prescribing information for solution (3) CLENPIQ. -------------------------------CONTRAINDICATIONS------------------------------ ® • Patients with severe reduced renal impairment (creatinine clearance less CLENPIQ (sodium picosulfate, magnesium oxide, and anhydrous citric than 30 mL/minute) (4, 5.3, 8.6) acid) oral solution • Gastrointestinal (GI) obstruction or ileus (4) Initial U.S. Approval: 2012 • Bowel perforation (4) • ----------------------------RECENT MAJOR CHANGES-------------------------- Toxic colitis or toxic megacolon (4) Indications and Usage (1) 08/2019 • Gastric retention (4) Dosage and Administration (2.1) 10/2019 • Hypersensitivity to any of the ingredients in CLENPIQ (4) Dosage and Administration (2.2) 08/2019, 10/2019 Dosage and Administration, Day-Before Dosage Regimen (2.3) -----------------------WARNINGS AND PRECAUTIONS------------------------ Removed 10/2019 • Risk of fluid and electrolyte abnormalities, arrhythmia, seizures, and renal Warnings and Precautions (5.1) 08/2019 impairment: Encourage adequate hydration, assess concurrent medications, and consider laboratory assessments prior to and after use. (5.1, 5.2, 5.3, 5.4, ----------------------------INDICATIONS AND USAGE--------------------------- 7.1) CLENPIQ® is a combination of sodium picosulfate, a stimulant laxative, and • Use in patients with renal impairment or taking concomitant medications magnesium oxide and anhydrous citric acid, which form magnesium citrate, that affect renal function: Use caution, ensure adequate hydration, and an osmotic laxative, indicated for cleansing of the colon as a preparation for consider testing. -

Review of the Role of Mannitol in the Therapy of Children
18th Expert Committee on the Selection and Use of Essential Medicines (21 to 25 March 2011) Section 16: Diuretics -- Mannitol review (Children) Review of the role of mannitol in the therapy of children Dr Fatemeh Tavakkoli M.D. Pharm. D Candidate, University of Maryland, School of Pharmacy, Baltimore, Maryland, USA Contents Introduction......................................................................................................................................... 3 Background ......................................................................................................................................... 3 Table 1: available guidelines for Mannitol ................................................................................ 3 Public Health Burden.......................................................................................................................... 5 Table 2: Indications and dose regimens for Mannitol in children .............................................. 6 Search methods for identification of safety and efficacy data............................................................ 7 Results................................................................................................................................................. 8 Systematic reviews.............................................................................................................................. 8 Individual studies (categorized by therapeutic use of mannitol): ..................................................... 10 -

Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Laxatives for the management of constipation in people receiving palliative care (Review) Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 5 http://www.thecochranelibrary.com Laxatives for the management of constipation in people receiving palliative care (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 14 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 26 ADDITIONALTABLES. 26 APPENDICES ..................................... 28 WHAT’SNEW..................................... 35 HISTORY....................................... 35 CONTRIBUTIONSOFAUTHORS . 36 DECLARATIONSOFINTEREST . 36 SOURCESOFSUPPORT . 36 DIFFERENCES -

Jbscreen Membrane 1 (PEG 400 to PEG 2000 MME Based) Cat.-No.: CS-301L
JBScreen Membrane 1 (PEG 400 to PEG 2000 MME based) Cat.-No.: CS-301L No. Precipitant Precipitant 2 Buffer Additive A1 15 % w/v Polyethylene glycol 400 15 % w/v Glycerol 100 mM HEPES; pH 7.5 200 mM Calcium chloride A2 20 % w/v Polyethylene glycol 400 100 mM Sodium chloride 100 mM tri-Sodium citrate; pH 5.6 20 mM Magnesium chloride A3 25 % w/v Polyethylene glycol 400 none 50 mM Sodium acetate; pH 4.6 50 mM Magnesium acetate A4 30 % w/v Polyethylene glycol 400 50 mM Sodium sulfate 50 mM TRIS; pH 8.5 50 mM Lithium sulfate A5 48 % w/v Polyethylene glycol 400 none 100 mM HEPES; pH 7.5 200 mM Calcium chloride A6 20 % w/v Polyethylene glycol monomethyl ether 550 none 10 mM TRIS; pH 7.5 none A7 30 % w/v Polyethylene glycol monomethyl ether 550 none 50 mM TRIS; pH 8.5 100 mM Magnesium chloride A8 35 % w/v Polyethylene glycol 600 none none none A9 28 % w/v Polyethylene glycol 1,000 10 % w/v Glycerol 100 mM TRICINE; pH 8.0 350 mM Sodium chloride A10 10 % w/v Polyethylene glycol 1,500 5 % w/v Ethanol none 100 mM Magnesium chloride, 100 mM Sodium chloride A11 30 % w/v Polyethylene glycol 1,500 none none none A12 5 % w/v Polyethylene glycol 2,000 none none none B1 10 % w/v Polyethylene glycol 2,000 none 100 mM TRIS; pH 8.5 500 mM Magnesium chloride B2 15 % w/v Polyethylene glycol 2,000 none none none B3 15 % w/v Polyethylene glycol 2,000 none none 100 mM Lithium chloride B4 15 % w/v Polyethylene glycol 2,000 none 100 mM Sodium Phosphate; pH 6.2 20 mM tri-Sodium citrate B5 15 % w/v Polyethylene glycol 2,000 none 100 mM Sodium Phosphate; pH 6.8 500 mM -

Dental and Metabolic Effects of Lactitol in the Diet of Laboratory Rats
Downloaded from British Journal of Nutrition (1989), 61, 17-24 17 https://www.cambridge.org/core Dental and metabolic effects of lactitol in the diet of labolratory rats BY T. H. GRENBY AND A. PHILLIPS Department of Oral Medicine and Pathology, United Medical and Dental Schools, Guy’s Hospital, London SEI 9RT . IP address: (Received 17 May 1988 - Accepted 30 August 1988) 170.106.35.93 I. Because so little is known about the properties of lactitol as a possible alternative bulk sweetener to sucrose, it was tested in two large-scale experiments in laboratory rats. Matched groups of caries-active Osborne-Mendel rats were fed.on uniform diets containing lactitol and compared with a sucrose control in both experiments, plus a xylitol control in the first experiment. , on 2. In the early stages of the experiments weight gains and food utilization were better on the Sucrose than on 26 Sep 2021 at 05:12:03 the lactitol regimens. Body-fat storage was higher on the sucrose than on the polyol regimens. 3. At the end of 8 weeks the mandibular molars were examined for dental plaque accumulation and dental caries. The dental caries scores when 160 g sucrose/kg in the diet was replaced by lactitol were lower by a highly significant margin, bringing them down to the same low level as those on a 160 g xylitol/kg regimen. 4. Testing lactitol in a manufactured food product, shortbread biscuits, in comparison with ordinary sucrose biscuits, showed differences in plaque scores (significant) and caries levels (highly significant), with 60 % fewer lesions on the lactitol regimen. -

3.2.2 Misuse of Stimulant Laxatives
Medicines Adverse Reactions Committee Meeting date 10/06/2021 Agenda item 3.2.2 Title Misuse of stimulant laxatives Submitted by Medsafe Pharmacovigilance Paper type For advice Team Active ingredient Product name Sponsor Bisacodyl Bisacodyl Laxative (Pharmacy Health) PSM Healthcare Limited trading as API tablet Consumer Brands Dulcolax tablet Sanofi-Aventis New Zealand Limited Dulcolax Suppository Sanofi-Aventis New Zealand Limited *Lax-Suppositories Bisacodyl AFT Pharmaceuticals Limited *Lax-Tab tablet AFT Pharmaceuticals Limited Docusate sodium *Coloxyl tablet Pharmacy Retailing (New Zealand) Limited trading as Healthcare Logistics Docusate sodium + Coloxyl with Senna tablet Pharmacy Retailing (New Zealand) sennosides Limited trading as Healthcare Logistics *Laxsol tablet Pharmacy Retailing (New Zealand) Limited trading as Healthcare Logistics Glycerol *Glycerol Suppositories PSM Healthcare Limited trading as API Consumer Brands Sennosides *Senokot tablet Reckitt Benckiser (New Zealand) Limited Sodium picosulfate Dulcolax SP Drops oral solution Sanofi-Aventis New Zealand Limited PHARMAC funding *Pharmaceutical Schedule Lax-Tab tablets, Lax-Suppositories Bisacodyl, Coloxyl tablets and Glycerol Suppositories are fully-funded only on a prescription. Senokot tablets are part- funded. Previous MARC Misuse of stimulant laxatives has not been discussed previously. meetings International action Following a national safety review published in August 2020, the MHRA in the UK has introduced pack size restrictions, revised recommended ages for use