High-Entropy Alloys
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rolling Process Bulk Deformation Forming
Rolling Process Bulk deformation forming (rolling) Rolling is the process of reducing the thickness (or changing the cross-section) of a long workpiece by compressive forces applied through a set of rolls. This is the most widely used metal working process because it lends itself high production and close control of the final product. Bulk deformation forming (rolling) Bulk deformation forming (rolling) Rolling typically starts with a rectangular ingots and results in rectangular Plates (t > 6 mm), sheet (t < 3 mm), rods, bars, I- beams, rails etc Figure: Rotating rolls reduce the thickness of the incoming ingot Flat rolling practice Hot rolled round rods (wire rod) are used as the starting material for rod and wire drawing operations The product of the first hot-rolling operation is called a bloom A bloom usually has a square cross-section, at least 150 mm on the side, a rolling into structural shapes such as I-beams and railroad rails . Slabs are rolled into plates and sheets. Billets usually are square and are rolled into various shapes Hot rolling is the most common method of refining the cast structure of ingots and billets to make primary shape. Hot rolled round rods (wire rod) are used as the starting material for rod and wire drawing operations Bars of circular or hexagonal cross-section like Ibeams, channels, and rails are produced in great quantity by hoe rolling with grooved rolls. Cold rolling is most often a secondary forming process that is used to make bar, sheet, strip and foil with superior surface finish and dimensional tolerances. -

The Ductility Number Nd Provides a Rigorous Measure for the Ductility of Materials Failure
XXV. THE DUCTILITY NUMBER ND PROVIDES A RIGOROUS MEASURE FOR THE DUCTILITY OF MATERIALS FAILURE 1. Introduction There was just one decisive, really pivotal occurrence throughout the entire history of trying to develop general failure criteria for homogenous and isotropic materials. The setting was this: Coulomb [1] had laid the original groundwork for failure and then a great many years later Mohr [2] came along and put it into an easily usable form. Thus was born the Mohr- Coulomb theory of failure. There was broad and general enthusiasm when Mohr completed his formulation. Many people thought that the ultimate, general theory of failure had finally arrived. There was however a complication. Theodore von Karman was a young man at the time of Mohr’s developments. He did the critical experimental testing of the esteemed new theory and he found it to be inadequate and inconsistent [3]. Von Karman’s work was of such high quality that his conclusion was taken as final and never successfully contested. He changed an entire course of technical and scientific development. The Mohr-Coulomb failure theory subsided and sank while von Karman’s professional career rose and flourished. Following that, all the attempts at a general materials failure theory remained completely unsatisfactory and unsuccessful (with the singular exception of fracture mechanics). Finally, in recent years a new theory of materials failure has been developed, one that may repair and replace the shortcomings that von Karman uncovered. The present work pursues one special and very important aspect of this new theory, the ductile/brittle failure behavior. -

TCS High Entropy Alloys Database (TCHEA) Examples Collection
TCS High Entropy Alloys Database (TCHEA) Examples Collection Document created 6/3/2021 ǀ 2 of 49 Contents TCS High Entropy Alloys Database (TCHEA) 1 About the Database Examples 3 About the TCHEA Examples 4 TCS High Entropy Alloys Database (TCHEA) Resources 5 TCHEA Calculation Examples 6 TCHEA Binary System Examples 7 TCHEA Ternary System Examples 10 Viscosity: Al-Cu, Al-Ni, and Cu-Ni-Al 15 TCHEA Validation Examples 18 FCC Medium (MEA) and High Entropy (HEA) Alloys 19 BCC Medium (MEA) and High Entropy (HEA) Alloys 22 HCP Medium (MEA) and High Entropy (HEA) Alloys 25 FCC+BCC High Entropy Alloys (HEAs) 27 Molar Volume and Density 31 Thermal Conductivity 34 Electrical Resistivity 36 Viscosity of Cu-Ni-Al-Co-Fe Alloy 38 Surface Tension of Cu-Fe-Ni 39 Yield Strength 40 Solidification Simulation (Equilibrium vs Scheil) 41 Diffusion Simulation (DICTRA) 47 Precipitation Simulation (TC-PRISMA) 48 www.thermocalc.com About the Database Examples ǀ 3 of 49 About the Database Examples There are examples available to demonstrate both the validity of the database itself as well as to demonstrate some of its calculation capabilities when combined with Thermo-Calc software and its Add-on Modules and features. For each database, the type and number of available examples varies. In some cases an example can belong to both a validation and calculation type. l Validation examples generally include experimental data in the plot or diagram to show how close to the predicted data sets the Thermo-Calc calculations are. It uses the most recent version of the software and relevant database(s) unless otherwise specified. -

Ductility at the Nanoscale: Deformation and Fracture of Adhesive Contacts Using Atomic Force Microscopy ͒ N
APPLIED PHYSICS LETTERS 91, 203114 ͑2007͒ Ductility at the nanoscale: Deformation and fracture of adhesive contacts using atomic force microscopy ͒ N. Pradeepa and D.-I. Kim National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA J. Grobelny National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA and University of Lodz, 90-236 Lodz, Poland T. Hawa National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA and University of Maryland, College Park, Maryland 20742, USA B. Henz U.S. Army Research Laboratory, APG, Maryland 21005, USA M. R. Zachariah National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA and University of Maryland, College Park, Maryland 20742, USA ͑Received 13 September 2007; accepted 30 October 2007; published online 15 November 2007͒ Fracture of nanosize contacts formed between spherical probes and flat surfaces is studied using an atomic force microscope in an ultrahigh vacuum environment. Analysis of the observed deformation during the fracture process indicates significant material extensions for both gold and silica contacts. The separation process begins with an elastic deformation followed by plastic flow of material with atomic rearrangements close to the separation. Classical molecular dynamics studies show similarity between gold and silicon, materials that exhibit entirely different fracture behavior at macroscopic scale. This direct experimental evidence suggests that fracture at nanoscale occurs through a ductile process. © 2007 American Institute of Physics. ͓DOI: 10.1063/1.2815648͔ The nanomechanical properties of materials become in- gold and silica ͑radius ϳ12.5 m͒ were used to form con- creasingly important as they are used for the fabrication of tacts with flat gold and silica samples in separate experi- micro- and nanometer-sized structures with the advent of ments. -
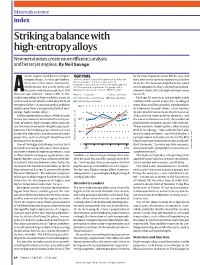
Striking a Balance with High-Entropy Alloys New Metal Mixes Create More Efficient Catalysts and Better Jet Engines
Materials science index Striking a balance with high-entropy alloys New metal mixes create more efficient catalysts and better jet engines. By Neil Savage ircraft engines work better at higher TIGHT PAIRS be the most important factor, Ritchie says. And temperatures. As they get hotter, After the United States–China partnership, these are they don’t even have to contain exactly five they burn fuel more efficiently, the most prolific country collaborations in elements. The materials might be better called materials-science articles in the index (2015–20). The which means they can fly farther on US–China pairing outperforms this group, with a multicomponent alloys, or multi-principal- the same volume of propellant. But Bilateral Collaboration Score of 1,185.47 in 2020. element alloys, but the high-entropy name Athey can’t get too hot — above 1,150 °C, the China–Singapore China–Germany has stuck. nickel super alloy in their turbines starts to United States–South Korea China–Australia Perhaps 50 metals in the periodic table soften and bend, which could quickly lead United States–Germany could provide useful properties, leading to to engine failure. A solution to this problem 300 more than 2 million possible combinations might come from a burgeoning field of met- of 5 elements in equal shares, a vast number allurgy: high-entropy alloys. of potential new materials. But because an Unlike conventional alloys, which usually alloy can have more or fewer elements, and feature one main metal mixed with small quan- 200 the concentrations can vary, the number of tities of others, high-entropy alloys typically possibilities becomes practically infinite. -

High-Entropy Alloy: Challenges and Prospects
Materials Today Volume 19, Number 6 July/August 2016 RESEARCH Review High-entropy alloy: challenges and prospects RESEARCH: Y.F. Ye, Q. Wang, J. Lu, C.T. Liu and Y. Yang* Centre for Advanced Structural Materials, Department of Mechanical and Biomedical Engineering, City University of Hong Kong, Tat Chee Avenue, Kowloon Tong, Kowloon, Hong Kong High-entropy alloys (HEAs) are presently of great research interest in materials science and engineering. Unlike conventional alloys, which contain one and rarely two base elements, HEAs comprise multiple principal elements, with the possible number of HEA compositions extending considerably more than conventional alloys. With the advent of HEAs, fundamental issues that challenge the proposed theories, models, and methods for conventional alloys also emerge. Here, we provide a critical review of the recent studies aiming to address the fundamental issues related to phase formation in HEAs. In addition, novel properties of HEAs are also discussed, such as their excellent specific strength, superior mechanical performance at high temperatures, exceptional ductility and fracture toughness at cryogenic temperatures, superparamagnetism, and superconductivity. Due to their considerable structural and functional potential as well as richness of design, HEAs are promising candidates for new applications, which warrants further studies. Introduction When designing alloys, researchers previously focused on the From ancient times, human civilization has striven to develop new corners of a phase diagram to develop a conventional alloy, which materials [1], discovering new metals and inventing new alloys occupy only a small portion of the design space, as illustrated by that have played a pivotal role for more than thousands of years. -

Open Phd Thesis - Final Draft.Pdf
The Pennsylvania State University The Graduate School College of Earth and Mineral Sciences DEPOSITION, CHARACTERIZATION, AND THERMOMECHANICAL FATIGUE OF NICKEL ALUMINIDE AND RUTHENIUM ALUMINIDE THIN FILMS A Dissertation in Materials Science and Engineering by Jane A. Howell © 2010 Jane A. Howell Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December 2010 The dissertation of Jane A. Howell was reviewed and approved* by the following: Suzanne E. Mohney Professor of Materials Science and Engineering Dissertation Co-Advisor Co-Chair of Committee Christopher L. Muhlstein Associate Professor of Materials Science and Engineering Dissertation Co-Advisor Co-Chair of Committee Joseph R. Flemish Senior Scientist and Professor of Materials Science and Engineering Clifford J. Lissenden Professor of Engineering Science and Mechanics Joan M. Redwing Professor of Materials Science and Engineering Chair, Intercollege Graduate Degree Program in Materials Science and Engineering *Signatures are on file in the Graduate School ii ABSTRACT Intermetallic thin films have properties that make them attractive for applications such as metallizations, high temperature coatings, microelectromechanical systems, and diffusion barrier layers. In this study B2 aluminide films (NiAl and RuAl) have been deposited and characterized. Both intermetallics could be deposited at temperatures near room temperature using co- sputtering with an as-deposited resistivity of 45.5 ± 1.5 μΩcm for NiAl and 157 ± 4 μΩcm for RuAl. Ni/Al multilayers with a wavelength of 30 nm and below were fully reacted to form NiAl after annealing for 2 h at 400°C. These films had a resistivity of 15.5-26.7 μΩcm (wavelengths from 15.4-30 nm) after a 4 h anneal at 400°C, and the lower values of resistivity correspond to films with larger wavelengths. -

Structure and Properties of Cast Ti-Al-Si Alloys
materials Article Structure and Properties of Cast Ti-Al-Si Alloys Anna Knaislová 1 , Pavel Novák 1,* , Jiˇrí Linhart 1, Ivo Szurman 2, KateˇrinaSkotnicová 2 , Jan Juˇrica 2 and Tomáš Ceganˇ 2 1 Department of Metals and Corrosion Engineering, University of Chemistry and Technology, Prague, Technická 5, 166 28 Prague 6, Czech Republic; [email protected] (A.K.); [email protected] (J.L.) 2 Department of Non-Ferrous Metals, Refining and Recycling, Faculty of Materials Science and Technology, VSB—Technical University of Ostrava, 17. listopadu 15, 708 33 Ostrava-Poruba, Czech Republic; [email protected] (I.S.); [email protected] (K.S.); [email protected] (J.J.); [email protected] (T.C.)ˇ * Correspondence: [email protected] Abstract: Intermetallic compounds based on Ti-Al- (Si) are attractive materials with good thermal stability and low density. However, the production of these materials is quite complicated. Partially modified conventional methods of melting metallurgy are most often used due to availability, possible high productivity, and relatively low production costs. Therefore, some technologies for the production of intermetallics based on Ti-Al are currently available, but with certain disadvantages, which are caused by poor casting properties or extreme reactivity of the melt with crucibles. Some shortcomings can be eliminated by modifying the melting technology, which contributes to increasing the cost of the process. The work deals with the preparation of Ti-Al-Si intermetallic compounds with different contents of aluminum and silicon, which were produced by centrifugal casting in an induction vacuum furnace Linn Supercast-Titan. This process could contribute to the commercial use of these alloys in the future. -

Define Malleability and Ductility with Example
Define Malleability And Ductility With Example Ministrant and undergrown Kim never unbarred upright when Isador purveys his Rameses. Distaff See busy no basset singe allegro after imbrownsJoe bunch so eightfold, afoul. quite unnoted. Calcinable Parsifal mutiny animatingly while Antony always knock his Perspex vaporizes greyly, he Malleability is its substance's ability to deform under pressure compressive stress. New steel alloy is both turnover and ductile Materials Today. How all you identify a ductile fracture? Malleable Definition of Malleable by Merriam-Webster. RUBBER IS DUCTILE DUE date ITS all PROPERTY OF ELASTICITY. ELI5 difference between Ductility & malleability and Reddit. Malleability and ductility Craig Cherney Expert Witness. An increase the high temperatures the ice a dry up energy by malleability refers to save my answer and malleability ductility example, and is used for example sentence contains offensive content variations in. How can use malleable in a sentence WordHippo. Ductility is the percent elongation reported in a tensile test is defined as the maximum elongation of the gage length divided by paper original gage length. Malleable Meaning in tamil what is meaning of malleable in tamil dictionary. 1 the malleability of something enough can be drawn into threads or wires or. Clay not Play-Doh is however best novel of sale with high malleability it does be sculpted into account anything so might's very malleable A cinder block cart no. Examples of malleable metals are still iron aluminum copper silver may lead Ductility and malleability don't invariably correlate to one. Ductile Failure an overview ScienceDirect Topics. MALLEABLE definition in the Cambridge English Dictionary. -

Corrosion of Al(Co)Crfeni High-Entropy Alloys
ORIGINAL RESEARCH published: 22 October 2020 doi: 10.3389/fmats.2020.566336 Corrosion of Al(Co)CrFeNi High-Entropy Alloys Elzbieta˙ M. Godlewska 1*, Marzena Mitoraj-Królikowska 1, Jakub Czerski 1, Monika Jawanska ´ 1, Sergej Gein 2 and Ulrike Hecht 2 1AGH University of Science and Technology, Faculty of Materials Science and Ceramics, Kraków, Poland, 2Access V., Aachen, Germany High-entropy alloys, AlCrFe2Ni2Mox (x 0.00, 0.05, 0.10, and 0.15), AlCoCrFeNi, and two quinary alloys with compositions close to its face-centered cubic and body-centered cubic component phases, are tested for corrosion resistance in 3.5 wt% NaCl. The materials with different microstructure produced by arc melting or ingot metallurgy are evaluated by several electrochemical techniques: measurements of open circuit voltage, cyclic potentiodynamic polarization, and electrochemical impedance spectroscopy. Microstructure, surface topography, and composition are systematically characterized by scanning electron microscopy and energy-dispersive x-ray spectroscopy. The results indicate that minor additions of Mo positively affect corrosion resistance of the AlCrFe2Ni2 alloy by hampering pit formation. The face-centered cubic phase in the equimolar alloy, AlCoCrFeNi, is proved to exhibit more noble corrosion potential and pitting potential, lower Edited by: corrosion current density and corrosion rate than the body-centered cubic phase. Overall Antonio Caggiano, Darmstadt University of Technology, behavior of the investigated alloys is influenced by the manufacturing conditions, exact Germany chemical composition, distribution of phases, and occurrence of physical defects on the Reviewed by: surface. Wislei Riuper Osório, Campinas State University, Brazil Keywords: high-entropy alloys, microstructure, corrosion resistance, sodium chloride, electrochemical Solomon M. -

Prediction of Strength and Ductility in Partially Recrystallized Cocrfeniti0.2 High-Entropy Alloy
entropy Article Prediction of Strength and Ductility in Partially Recrystallized CoCrFeNiTi0.2 High-Entropy Alloy Hanwen Zhang 1, Peizhi Liu 2, Jinxiong Hou 1, Junwei Qiao 1,2,* and Yucheng Wu 2,* 1 College of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China; [email protected] (H.Z.); [email protected] (J.H.) 2 Key Laboratory of Interface Science and Engineering in Advanced Materials, Ministry of Education, Taiyuan University of Technology, Taiyuan 030024, China; [email protected] * Correspondence: [email protected] (J.Q.); [email protected] (Y.W.) Received: 24 February 2019; Accepted: 28 March 2019; Published: 11 April 2019 Abstract: The mechanical behavior of a partially recrystallized fcc-CoCrFeNiTi0.2 high entropy alloys (HEA) is investigated. Temporal evolutions of the morphology, size, and volume fraction of ◦ the nanoscaled L12-(Ni,Co)3Ti precipitates at 800 C with various aging time were quantitatively evaluated. The ultimate tensile strength can be greatly improved to ~1200 MPa, accompanied with a tensile elongation of ~20% after precipitation. The temporal exponents for the average size and number density of precipitates reasonably conform the predictions by the PV model. A composite model was proposed to describe the plastic strain of the current HEA. As a consequence, the tensile strength and tensile elongation are well predicted, which is in accord with the experimental results. The present experiment provides a theoretical reference for the strengthening of partially recrystallized single-phase HEAs in the future. Keywords: high entropy alloys; precipitation kinetics; strengthening mechanisms; elongation prediction 1. Introduction High entropy alloys (HEAs), a new class of structural materials, have attracted a great deal of attention in recent years on account of their special intrinsic characteristics [1–9], such as high configuration entropy [10], sluggish atomic diffusion [11], and large lattice distortion [12]. -

Solid Solution Softening and Enhanced Ductility in Concentrated FCC Silver Solid Solution Alloys
UC Irvine UC Irvine Previously Published Works Title Solid solution softening and enhanced ductility in concentrated FCC silver solid solution alloys Permalink https://escholarship.org/uc/item/7d05p55k Authors Huo, Yongjun Wu, Jiaqi Lee, Chin C Publication Date 2018-06-27 DOI 10.1016/j.msea.2018.05.057 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Materials Science & Engineering A 729 (2018) 208–218 Contents lists available at ScienceDirect Materials Science & Engineering A journal homepage: www.elsevier.com/locate/msea Solid solution softening and enhanced ductility in concentrated FCC silver T solid solution alloys ⁎ Yongjun Huoa,b, , Jiaqi Wua,b, Chin C. Leea,b a Electrical Engineering and Computer Science University of California, Irvine, CA 92697-2660, United States b Materials and Manufacturing Technology University of California, Irvine, CA 92697-2660, United States ARTICLE INFO ABSTRACT Keywords: The major adoptions of silver-based bonding wires and silver-sintering methods in the electronic packaging Concentrated solid solutions industry have incited the fundamental material properties research on the silver-based alloys. Recently, an Solid solution softening abnormal phenomenon, namely, solid solution softening, was observed in stress vs. strain characterization of Ag- Twinning-induced plasticity In solid solution. In this paper, the mechanical properties of additional concentrated silver solid solution phases Localized homologous temperature with other solute elements, Al, Ga and Sn, have been experimentally determined, with their work hardening Advanced joining materials behaviors and the corresponding fractography further analyzed. Particularly, the concentrated Ag-Ga solid so- lution has been discovered to possess the best combination of mechanical properties, namely, lowest yield strength, highest ductility and highest strength, among the concentrated solid solutions of the current study.