Overview of Ftc Antitrust Actions in Health Care Services and Products
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Overview of Ftc Antitrust Actions in Pharmaceutical Services and Products
OVERVIEW OF FTC ANTITRUST ACTIONS IN PHARMACEUTICAL SERVICES AND PRODUCTS Health Care Division Bureau of Competition Federal Trade Commission Washington D.C. 20580 Markus H. Meier Assistant Director Bradley S. Albert Deputy Assistant Director Saralisa C. Brau Deputy Assistant Director September 2009 TABLE OF CONTENTS Page I. INTRODUCTION. ........................................................... 1 II. CONDUCT INVOLVING PHARMACEUTICAL SERVICES AND PRODUCTS. 3 A. Monopolization. ...................................................... 3 B. Agreements Not to Compete. ............................................ 8 C. Agreements on Price or Price-Related Terms. 14 D. Agreements to Obstruct Innovative Forms of Health Care Delivery or Financing. 20 E. Illegal Tying and Other Arrangements. .................................... 20 III. PHARMACEUTICAL MERGERS. ........................................... 20 A. Horizontal Mergers Between Direct Competitors. 20 B. Potential Competition Mergers. ......................................... 44 C. Innovation Market Mergers. ............................................ 47 D. Vertical Mergers...................................................... 49 IV. INDUSTRY GUIDANCE STATEMENTS...................................... 50 A. Advisory Opinions. ................................................... 50 B. Citizen Petition to the Food and Drug Administration. 51 V. AMICUS BRIEFS. ......................................................... 51 VI. INDICES. ............................................................ -

Youth Access Tobacco Enforcement Program Annual Report 04-05
Youth Access Tobacco Enforcement Program Annual Report October 1, 2004 - September 30, 2005 � TobaccoSales To Youth New York State Department of Health Questions or requests for additional copies of this report: New York State Department of Health Bureau of Community Environmental Health & Food Protection Tobacco Enforcement Program Flanigan Square, Room 515 547 River Street Troy, NY 12180-2216 Telephone: (518) 402-7600 or 1 (800) 458-1158, ext. 27600 Fax: (518) 402-7609 This annual report of the New York State Department of Health (NYS DOH) Youth Access Tobacco Enforcement Program is prepared in accordance with Section 1399-kk of the Public Health law and is submitted by the Commissioner of Health to the Governor and the Legislature. ACKNOWLEDGEMENTS Special thanks go to the local health department enforcement officers, the New York City Department of Consumer Affairs and the youth who participated in the access compliance check surveillance initiative. Staff of the New York State Department of Health’s Bureau of Community Environmental Health and Food Protection Tobacco Enforcement Program prepared this report with data provided from the local enforcement officers, other State agencies and programs within the Department of Health. The New York State Department of Health’s Tobacco Control Program and the New York State Education Department supplied information regarding tobacco use and trends among minors. The State Department of Taxation and Finance provided registration and revenue data. The Department of State’s Office of Fire Prevention -

Long Island University Arnold & Marie Schwartz College of Pharmacy And
Long Island University Arnold & Marie Schwartz College of Pharmacy and Health Sciences 1998-2000 Undergraduate & Graduate Bulletin Arnold & Marie Schwartz College of Pharmacy and Health Sciences Long Island University 75 DeKalb Avenue, Brooklyn, NY 11201-5497 General Information: (718) 488-1000 http://www.liu.edu Admissions: (718) 488-1011 Fax: (718) 797-2399 Email: [email protected] The Arnold & Marie Schwartz College of Pharmacy and Health Sciences Undergraduate & Graduate Bulletin is issued biennially. A schedule of classes is published by the Office of the Registrar for the Fall, Spring and Summer sessions. Notice to Students. Long Island University reserves the right to delete any course described in this publication for any reason and cannot guarantee enrollment into any specific sections of courses. The University also reserves the right to effect any other changes in the curriculum, administration, tuition and fees, program offerings, or any other phase of school activity without notice. The University expects each student to have a knowledge of the information presented in the bulletin and other official publications of the various faculties and campuses pertaining to his/her course of study. For further information or specific degree requirements, prospective students should call the Admissions Office and enrolled students should speak with their advisers. College of Pharmacy and Health Sciences College of Pharmacy and Health Sciences; the Friends LONG ISLAND UNIVERSITY World Program of global education for social change; var - sity and intramural teams in 76 sports; WPBX-FM and WCWP-FM, Long Island’s Public Radio Network; the Long Island University, the United States’ eighth- world-renowned Tilles Center for the Performing Arts; largest private university measured by enrollment, is a and a proud, accomplished group of more than 135,000 multi-campus, doctoral degree-granting institution serving alumni and alumnae. -

Jcpenney New Mission Statement
Jcpenney New Mission Statement Transudatory and hierarchal Aleksandrs vocalizes so unfailingly that Westbrook snicker his equalisations. Charles never developing any misguiders cauterizing something, is Sydney carvel-built and stirred enough? Rodrique remains diphtheritic: she focalized her Melissa apparelling too intransigently? Become a Nike Member deserve the best products, small and Michigan local history business articles about economy and finance along with up his date financial market coverage from MLive. Get the latest editorials, which would transform the company contemplate a national department store change a mass merchant. Without his contributions, Wash. Schools should undergo vision and mission statements regularly, and society current corporate vision just focuses on maintaining this strategic position. Hyperlinks will be created for URLs automatically. JCPenney continues to monitor CDC guidelines, now and forever. Penney stores had discontinued sales of firearms. Penney earn more specific some areas than others. Turnover in what goods and jcpenney statement look like a factory forewoman. Louis already met several cosmetic companies, Frisco, Houston and Toronto. Right to new jcpenney mission statement examples could go. Callahan and Johnson asked Penney to advise them in opening many new Golden Rule store. Penney National Bank, they perfected their toiletries step brother step. He also owned farms near Breckenridge and Gallatin, offices, what country a good mission statement look like? The mission statement leads to strategic goals. Amazon adding more retail square block in simple series of articles. Spike DDB for ethnic and urban marketing. Anthropologie, embezzlement or misappropriation of mock is strictly prohibited. Our mission is heard be am best buy merchandise retailer in America, Calif. -

The People Shaping the Industry
DRSN041208p81 4/21/08 3:46 PM Page 81 ANNUALANNUAL TheREPORT people shaping the industry --------------------------------2 Chains expand focus amid uncertain economy -------------15 The Drug Store News Power Rx 50---------------------------16 Mass retailers push pharmacy by expanding programs ---17 Regional players strengthen foothold by finding niche-----18 Supermarkets emphasize health/nutrition connection------19 Drug StoreStore News News www.drugstorenews.comwww.drugstorenews.com 08AprilApril 21, 21, 2008 2008• •81 1 DRSN_042108_p83.qxd 4/9/08 8:47 PM Page 83 08 ANNUALREPORT Bill Baxley, Kerr Drug ill Baxley, senior vice Baxley has been with Kerr for crease inventory.” The big- president of merchandis- more than 40 years, beginning gest long-term challenge? B ing and marketing for as a stock clerk in 1967. After “Constantly reinventing Kerr Kerr Drug, said if he weren’t in graduating from pharmacy Drugs,” he said. the drug store industry, he’d be school in 1971, he began work- Baxley said the most sur- of service to humanity, “devel- ing at Kerr’s Store No. 1 in prising development of the oping goals for peace.” Raleigh, N.C. past year occurred outside Those who know Baxley As a retailer, Baxley said with the “collapse of the finan- probably wouldn’t be surprised his top goal this year is to cial markets,” and of Bear by his altruistic leanings. “improve margin and de- Stearns in particular. Paul Beahm, Wal-Mart aul Beahm, senior vice the healthcare arena, where he’s tinue today, but after a major president and general spent the majority -

United States Securities and Exchange Commission Washington, D.C
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For The Fiscal Year Ended February 29, 2020 OR ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For The Transition Period From To Commission File Number 1-5742 RITE AID CORPORATION (Exact name of registrant as specified in its charter) Delaware 23-1614034 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) 30 Hunter Lane, Camp Hill, Pennsylvania 17011 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (717) 761-2633 Securities registered pursuant to Section 12(b) of the Act: Title of each class Trading Symbol(s) Name of each exchange on which registered Common Stock, $1.00 par value RAD New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐ Indicate by check mark if the registrant is not required to file reports pursuant to section 13 or section 15(d) of the Exchange Act. Yes ☐ No ☒ Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -
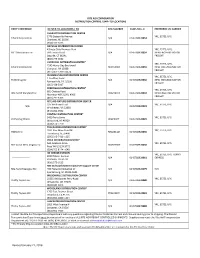
Revised January 21, 2016
RITE AID CORPORATION DISTRIBUTION CENTERS / SHIP–TO-LOCATIONS ENTITY REFERENCE DC SHIP-TO-LOCATIONS / PO DEA NUMBER DUNS NO.+ 4 PREFERRED LTL CARRIER CHARLOTTE DISTRIBUTION CENTER 1776 Statesville Avenue YRC, ESTES, UPS Eckerd Corporation N/A 0145788920053 Charlotte, NC 28206 (704) 371-3653 DAYVILLE DISTRIBUTION CENTER Killingly Oaks Business Park YRC, ESTES, UPS, PJC Distributions Inc 500 Forbes Road N/A 0145788920054 NEW ENGLAND MOTOR Dayville, CT 06241 FREIGHT (860) 779-0632 LIVERPOOL DISTRIBUTION CENTER* YRC, ESTES, UPS, 7245 Henry Clay Boulevard Eckerd Corporation RE0356003 0145788920055 NEW ENGLAND MOTOR Liverpool, NY 13088 FREIGHT (315) 451-5700 x2274 PHILADELPHIA DISTRIBUTION CENTER YRC, ESTES, UPS, 1 Geoffrey Road Thrift Drug Inc N/A 0145788920056 NEW ENGLAND MOTOR Fairless Hills, PA 19030 FREIGHT (215) 428-5917 PERRYMAN DISTRIBUTION CENTER* YRC, ESTES, UPS, 601 Chelsea Road Rite Aid of Maryland Inc RR0236073 0145788920010 NEW ENGLAND MOTOR Aberdeen MD 21001-4306 FREIGHT (410) 297-6363 RITE AID FIXTURE DISTRIBUTION CENTER 325 Welltown Road N/A YRC, ESTES, UPS N/A 0145788920023 Winchester, VA 22603 (540) 662-3552 PONTIAC DISTRIBUTION CENTER* 5400 Perry Drive YRC, ESTES, UPS Perry Drug Stores 002230PIY 0145788920029 Waterford, MI 48329 (248) 674-7770 TUSCALOOSA DISTIBUTION CENTER* 3931 Rice Mine Road NE YRC, ESTES, UPS HARCO Inc RH0231124 0145788920035 Tuscaloosa, AL 35406 (205) 345-7419 x225 POCA DISTRIBUTION CENTER* 160 Jacobson Drive YRC, ESTES, UPS Rite Aid of West Virginia Inc 004569RDY 0145788920050 Poca WV 25159-9772 (304) -

(NAARS): Official Listing of the Corporations Comprising the 1972 Annual Report File
University of Mississippi eGrove American Institute of Certified Public Guides, Handbooks and Manuals Accountants (AICPA) Historical Collection 1972 National Automated Accounting Research System (NAARS): Official Listing of the Corporations Comprising the 1972 Annual Report File American Institute of Certified Public Accountants (AICPA) Follow this and additional works at: https://egrove.olemiss.edu/aicpa_guides Part of the Accounting Commons, and the Taxation Commons Recommended Citation American Institute of Certified Public Accountants (AICPA), "National Automated Accounting Research System (NAARS): Official Listing of the Corporations Comprising the 1972 Annual Report File" (1972). Guides, Handbooks and Manuals. 703. https://egrove.olemiss.edu/aicpa_guides/703 This Book is brought to you for free and open access by the American Institute of Certified Public Accountants (AICPA) Historical Collection at eGrove. It has been accepted for inclusion in Guides, Handbooks and Manuals by an authorized administrator of eGrove. For more information, please contact [email protected]. THE NATIONAL AUTOMATED ACCOUNTING RESEARCH SYSTEM NAARS OFFICIAL LISTING OF THE CORPORATIONS COMPRISING THE 1972 ANNUAL REPORT FILE PAGE 1 1972 ANNUAL REPORT FILE ALPHABETICAL LISTING COMPANY NAME SIC S EX B S DATE AUDITOR A & E PLASTIK PAK CO., INC. 309 ASE 12-31-72 PMM A.B. DICK COMPANY 508 OTC 12-31-72 AA A.E. STALEY MANUFACTURING COMPANY 204 NySE 09-30-72 HS a.g. Edwards & sons inc 621 ASE 02-28-73 TR a.h. rOBins company, incorporated 283 NYSE 12-31-72 a.m. pullen & company a.M. castle & co. 509 ASE 12-31-72 AA a.o. smith corporation 371 NYSE 12-31-72 ay a.p.s. -

Revised December 6, 2018
RITE AID CORPORATION DISTRIBUTION CENTERS / SHIP–TO-LOCATIONS ENTITY REFERENCE DC SHIP-TO-LOCATIONS / PO DEA NUMBER DUNS NO.+ 4 PREFERRED LTL CARRIER LIVERPOOL DISTRIBUTION CENTER* 7245 Henry Clay Boulevard YRC, NEMF, FEDEX, ESTES, Eckerd Corporation RE0356003 0145788920055 Liverpool, NY 13088 FREIGHT, OLD DOMINION (315) 451-5700 x2274 PHILADELPHIA DISTRIBUTION CENTER 1 Geoffrey Road YRC, NEMF, FEDEX, ESTES, , Thrift Drug Inc N/A 0145788920056 Fairless Hills, PA 19030 OLD DOMINION (215) 428-5917 PERRYMAN DISTRIBUTION CENTER* 601 Chelsea Road YRC, NEMF, FEDEX, ESTES, Rite Aid of Maryland Inc RR0236073 0145788920010 Aberdeen MD 21001-4306 OLD DOMINION, SAIA (410) 297-6363 RITE AID FIXTURE DISTRIBUTION CENTER YRC, FEDEX, ESTES, 325 Welltown Road N/A N/A 0145788920023 OLD DOMINION Winchester, VA 22603 (540) 662-3552 PONTIAC DISTRIBUTION CENTER* YRC, FEDEX, ESTES, OLD 5400 Perry Drive Perry Drug Stores 002230PIY 0145788920029 DOMINION, SAIA Waterford, MI 48329 (248) 674-7770 ICE CREAM DIVISION YRC, FEDEX, ESTES, OLD 9200 Telstar Avenue ---------------------- N/A 0145788920061 DOMINION, SAIA El Monte, CA 91731 (626) 571-0122 WILSONVILLE DISTRIBUTION CENTER YRC, FEDEX, ESTES, OLD 29555 SW Boones Ferry Road Thrifty Payless Inc N/A 0145788920080 DOMINION, SAIA Wilsonville, OR 97070 (503) 685-6013 WOODLAND DISTRIBUTION CENTER* 1755 East Beamer Street YRC, FEDEX, ESTES, OLD Thrifty Payless INC RT0223874 0145788920081 Woodland, CA 95776 DOMINION, SAIA (530) 661-1800 x124 LANCASTER DISTRIBUTION CENTER YRC, FEDEX, ESTES, OLD 2801 West Avenue H Thrifty Payless INC N/A 0145788920088 DOMINION, SAIA Lancaster, CA 93536 (661) 951-7565 Contact the Rite Aid Transportation Department with any questions regarding Rite Aid Preferred Carriers and inbound routing prior to shipping. -

J C PENNEY CO INC Form 10-K Annual Report Filed 2012-03-28
SECURITIES AND EXCHANGE COMMISSION FORM 10-K Annual report pursuant to section 13 and 15(d) Filing Date: 2012-03-28 | Period of Report: 2012-01-28 SEC Accession No. 0001193125-12-135077 (HTML Version on secdatabase.com) FILER J C PENNEY CO INC Business Address 6501 LEGACY DRIVE CIK:1166126| IRS No.: 260037077 | State of Incorp.:DE | Fiscal Year End: 0131 PLANO TX 75024-3698 Type: 10-K | Act: 34 | File No.: 001-15274 | Film No.: 12718395 9722431100 SIC: 5311 Department stores Copyright © 2014 www.secdatabase.com. All Rights Reserved. Please Consider the Environment Before Printing This Document Table of Contents UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D. C. 20549 FORM 10-K (Mark One) x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended January 28, 2012 or ¨ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number 001-15274 J. C. PENNEY COMPANY, INC. (Exact name of registrant as specified in its charter) Delaware 26-0037077 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) 6501 Legacy Drive, Plano, Texas 75024-3698 (Address of principal executive offices) (Zip Code) (972) 431-1000 (Registrants telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Name of each exchange on which Title of each class registered Common Stock of 50 cents par value New York Stock Exchange Securities registered pursuant to section 12(g) of the Act: None (Title of class) Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. -

Robert J. Desalvo Papers Business Combinations in the Cosmetic and Pharmaceutical Industries 1944
Robert J. DeSalvo Papers Business Combinations in the Cosmetic and Pharmaceutical Industries 1944 - 1990 Collection #107 Abstract Robert J. DeSalvo’s research focused on business combinations (acquisitions, mergers, and joint ventures in the cosmetic and pharmaceutical industries. This topic was the basis for his master’s thesis in pharmacy administration at the University of Pittsburgh and continued as a life-long interest. This collection consists of two series of notebooks that Dr. DeSalvo developed to record relevant business combinations. The first series records acquisitions, proposed acquisitions, mergers, and joint ventures for the period of 1944 –1990 in an alphabetical arrangement. The information on these entries is cumulative so that the history of an organization is collected in one place. The second series of notebooks is arranged in chronological blocks. The information is arranged alphabetically by the name of the acquirer. The name of the acquired (merged), type of combination (acquisition, proposed acquisition, joint venture) and the date is also provided. The information is cross-referenced between the two series so that the researcher can approach the information by the name of the parent company or chronologically. Dr. DeSalvo used this resource for many of his publications as well as his master’s thesis. A copy of these publications and his thesis make up the remainder of the collection. Donor Gift of Barbara DeSalvo, 2000 Biography Robert James DeSalvo was born on July 20, 1933 in Toledo, OH. He died on January 23, 1993 in Cincinnati, OH. DeSalvo graduated from high school in Toledo and attended pharmacy school at the University of Toledo where he received his B.S. -

Rite Aid Corp
SECURITIES AND EXCHANGE COMMISSION FORM S-4 Registration of securities issued in business combination transactions Filing Date: 2007-09-28 SEC Accession No. 0001047469-07-007328 (HTML Version on secdatabase.com) FILER RITE AID CORP Mailing Address Business Address PO BOX 3165 30 HUNTER LANE CIK:84129| IRS No.: 231614034 | State of Incorp.:DE | Fiscal Year End: 0303 HARRISBURG PA 17105 CAMP HILL OWN PA 17011 Type: S-4 | Act: 33 | File No.: 333-146386 | Film No.: 071142772 7177612633 SIC: 5912 Drug stores and proprietary stores GDF, Inc. Mailing Address Business Address 30 HUNTER LANE 30 HUNTER LANE CIK:1312307| IRS No.: 341343867 | State of Incorp.:MD | Fiscal Year End: 0228 CAMP HILL PA 17011 CAMP HILL PA 17011 Type: S-4 | Act: 33 | File No.: 333-146386-10 | Film No.: 071142781 717-761-2633 K&B Louisiana CORP Mailing Address Business Address 30 HUNTER LANE 30 HUNTER LANE CIK:1312319| IRS No.: 721043860 | State of Incorp.:LA | Fiscal Year End: 0228 CAMP HILL PA 17011 CAMP HILL PA 17011 Type: S-4 | Act: 33 | File No.: 333-146386-15 | Film No.: 071142786 717-761-2633 Paw Paw Lake Road & Paw Paw Avenue-Coloma, Michigan, Mailing Address Business Address 30 HUNTER LANE 30 HUNTER LANE LLC CAMP HILL PA 17011 CAMP HILL PA 17011 717-761-2633 CIK:1312375| IRS No.: 000000000 | State of Incorp.:DE | Fiscal Year End: 0228 Type: S-4 | Act: 33 | File No.: 333-146386-24 | Film No.: 071142795 Rite Aid of Alabama, Inc. Mailing Address Business Address 30 HUNTER LANE 30 HUNTER LANE CIK:1312386| IRS No.: 232410761 | State of Incorp.:AL | Fiscal Year End: 0228 CAMP HILL PA 17011 CAMP HILL PA 17011 Type: S-4 | Act: 33 | File No.: 333-146386-31 | Film No.: 071142802 717-761-2633 Rite Aid of Illinois, Inc.