American Foundation for Pharmaceutical Education Box: 8
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identifying the Defendant in Products Liability Litigation: Alternate Theories for Compensating Injured Plaintiffs
International Journal of Business and Social Science Vol. 9 • No. 8 • August 2018 doi:10.30845/ijbss.v9n8p2 Identifying the Defendant in Products Liability Litigation: Alternate Theories for Compensating Injured Plaintiffs Richard J. Hunter, Jr. Professor of Legal Studies Seton Hall University United States John H. Shannon Professor of Legal Studies Seton Hall University United States Henry J. Amoroso Associate Professor of Legal Studies Seton Hall University United States Abstract A spirited public policy debate has raged in the United States for more than four decades revolving around the responsibility of drug manufacturers for their products that cause injury to unsuspecting plaintiffs. An important part of the debate centers around a drug—DES—that was marketed as a benefit to women in preventing miscarriages. Some interesting or "alternate" theories for assessing liability have received attention in the generic area of products liability. This article will discuss three of the most prominent and still controversial theories—alternative liability, enterprise liability, and market share liability. In addition, the article will include several case studies exemplifying the legal principles discussed — some coming to very difficult conclusions — regarding the efficacy and application of these theories to particular facts developed during litigation. Keywords: Alternative Liability; Enterprise Liability; Market Share Liability 1. Introduction DES (Diethylstilbestrol, a synthetic form of the female hormone estrogen) litigation in the United States has been both extensive and controversial. Forty years ago, at the height of the DES crisis, in a seminal law review article in the Fordham Law Review, Sheiner (1978) estimated that the number of women who took DES during pregnancy ranged from 1 1/2 million to 3 million. -

Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should Be Applied to the Sellers of Pharmaceutical Products? Richard C
Kentucky Law Journal Volume 78 | Issue 4 Article 3 1990 Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should be Applied to the Sellers of Pharmaceutical Products? Richard C. Ausness University of Kentucky Follow this and additional works at: https://uknowledge.uky.edu/klj Part of the Consumer Protection Law Commons, and the Food and Drug Law Commons Right click to open a feedback form in a new tab to let us know how this document benefits you. Recommended Citation Ausness, Richard C. (1990) "Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should be Applied to the Sellers of Pharmaceutical Products?," Kentucky Law Journal: Vol. 78 : Iss. 4 , Article 3. Available at: https://uknowledge.uky.edu/klj/vol78/iss4/3 This Article is brought to you for free and open access by the Law Journals at UKnowledge. It has been accepted for inclusion in Kentucky Law Journal by an authorized editor of UKnowledge. For more information, please contact [email protected]. Kentucky Law Journal Volume 78 1989-90 Number 4 Unavoidably Unsafe Products and Strict Products Liability: What Liability Rule Should be Applied to the Sellers of Pharmaceutical Products? BY RICHARD C. AusNEss* Table of Contents INTRODUCTION ....................................... 706 I. OVERVIEW ................................ 709 A. Strict Products Liability .............................. 709 B. Comment K and "Unavoidably Unsafe" Products ................................................... 712 II. COM[ENT K's LIABILITY REGm .......................... 719 A. Risks Associated with the Production Process. 720 B. Risks Arising from the Inherent Nature of a Product .................................................... 723 C. Risks Created by Conscious Design Choices .... 726 D. Scientifically Unknowable Risks ................... 731 E. -

Cholesterol How Kos Beat Big Pharma to HDL
The Other Cholesterol How Kos Beat Big Pharma to HDL www.pharmexec.com | ANADVANSTAR★ PUBLICATION Kos CEO Adrian Adams PHARMACEUTICAL EXECUTIVE Contents OCTOBER 2004 www.pharmexec.com Cover Story Under Construction Patrick Clinton Editor-in-Chief How Kos Pharmaceuticals turned a 50-year-old drug for raising HDL cholesterol into a brand-new, burgeoning business. Mulling the Future Kos CEO Adrian Adams, seated, maps strategy with key executives: (from left) Ralf Rosskamp, Christopher Kiritsy, Richard King, and Mark McGovern “We didn’t start with the idea of being a $200 million company. Growing as fast as possible: That was our goal from the get-go.” Kos Pharmaceuticals was first to market with a product to raise HDL cholesterol, and it wants to join pharma’s billion-dollar club by 2007. Big plan. Under Here’s how it’s going. Construction t’s hard to imagine that many pharma companies experienced more plain old good news this summer than Kos Pharmaceuticals. First, there were the second-quarter financials. Revenues for the quarter were $120.3 million, almost double last year’s figure. Net income for the quarter grew 245 percent to $38.5 million. The company raised its guidance for the year, predicting that full-year sales would be $480 million, 60 percent over last year— and CEO Adrian Adams boasted that Kos was the fastest-growing specialty pharma company in the United States. A few weeks later came encouraging results from a Phase II trial of IKos’ new inhalable insulin product for diabetes, and recruitment began for pivotal trials of a new combination product to consolidate Kos’ position in the burgeoning cholesterol market. -

V. : Civil Action No. DKC 14-3607
Case 8:14-cv-03607-DKC Document 47 Filed 07/29/15 Page 1 of 73 IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MARYLAND : MALLINCKRODT INC. : v. : Civil Action No. DKC 14-3607 : UNITED STATES FOOD AND DRUG ADMINISTRATION, et al. : MEMORANDUM OPINION Plaintiff Mallinckrodt Inc. (“Mallinckrodt”), a pharmaceutical manufacturer, brings this action seeking judicial review of actions taken by the United States Food and Drug Administration (“FDA”), regarding its methylphenidate hydrochloride extended-release tablets, a generic version of the brand-name drug Concerta. Presently pending and ready for review in this action are several motions: (1) a motion to dismiss filed by Defendants FDA and United States of America (“Defendants”) (ECF No. 30); (2) a motion to compel production of the administrative record filed by Mallinckrodt (ECF No. 31); (3) a motion for summary judgment filed by Mallinckrodt (ECF No. 34); and (2) two motions to seal filed by Mallinckrodt (ECF Nos. 4 and 20). The relevant issues have been briefed, and the court now rules, no hearing being deemed necessary. Local Rule 105.6. For the following reasons, Defendants’ motion to dismiss will be granted in part, and summary judgment will be granted in part Case 8:14-cv-03607-DKC Document 47 Filed 07/29/15 Page 2 of 73 against Plaintiff. Mallinckrodt’s motion to compel production of the administrative record will be denied as moot, and Mallinckrodt’s motions to seal will be denied. I. Background A. Statutory and Regulatory Background The FDA regulates the approval, manufacture, sale, and labeling of prescription drugs. -

05/01/02 Louisiana Medicaid Management
APPENDIX C 05/01/02 LOUISIANA MEDICAID MANAGEMENT INFORMATION SYSTEM PAGE 1 DEPT OF HEALTH AND HOSPITALS - BUREAU OF HEALTH SERVICES FINANCING LOUISIANA MEDICAID PHARMACY BENEFITS MANAGEMENT UNIT ONLY THESE COMPANIES PRODUCTS ARE COVERED AND ONLY THOSE DOSAGE FORMS LISTED IN APPENDIX A. MEDICAID DRUG FEDERAL REBATE PARTICIPATING PHARMACEUTICAL COMPANIES LABELER PHARMACEUTICAL COMPANY EFFECTIVE END DATE CODE DATE 00002 ELI LILLY & CO 04/01/91 00003 E.R.SQUIBB & SONS,INC 04/01/91 00004 HOFFMAN-LA ROCHE,INC 04/01/91 00005 LEDERLE LABORATORIES AMERICAN CYANAMID 04/01/91 00006 MERCK SHARP & DOHME 04/01/91 00007 SMITHKLINE BEECHAM CORPORATION 04/01/91 00008 WYETH LABORATORIES 04/01/91 00009 THE UPJOHN COMPANY 04/01/91 00011 BECTON DICKINSON MICROBIOLOGY SYSTEMS 10/01/91 07/01/98 00013 ADRIA LABORATORIES DIV.OF ERBAMONT,INC 04/01/91 00014 G.D.SEARLE & CO 04/01/91 01/01/01 00015 MEAD JOHNSON & COMPANY 04/01/91 00016 KABI PHARMACIA 04/01/91 00021 REED & CARNRICK 10/01/96 01/01/97 00023 ALLERGAN,INC 04/01/91 00024 WINTHROP PHARMACEUTICALS 04/01/91 00025 G.D.SEARLE & CO 04/01/91 00026 MILES INC.,PHARMACEUTICAL DIVISION 04/01/91 00028 GEIGY PHARMACEUTICALS 04/01/91 00029 SMITHKLINE BEECHAM CORPORATION 04/01/91 00031 ROBINS,A.H. 04/01/91 00032 SOLVAY PHARMACEUTICALS 04/01/91 00033 SYNTEX 04/01/91 00034 THE PURDUE FREDERICK COMPANY 04/01/91 00037 CARTER-WALLACE,INC 04/01/91 00038 STUART PHARMACEUTICALS,ICI AMERICAS INC 04/01/91 07/01/01 00039 HOECHST-ROUSSEL PHARMACEUTICALS INC 04/01/91 00043 SANDOZ CONSUMER CORPORATION 04/01/91 00044 KNOLL PHARMACEUTICALS -

Health Industry Business Communications Council
Health Industry Business Communications Council Registered Labelers: Accredited Auto-ID Labeling Standards Argentina New MedTek Devices Pty Ltd Oxavita SRL Norseld Pty Ltd. Novadien Healthcare Pty Ltd The following companies Odontit S.A. (and/or their subsidiaries/ PAMPAMED S.R.L. Numedico Technologies Pty Ltd divisions) have applied PATEJIM SRL Opto Global Pty. Ltd. for a Labeler Identification Orthocell Limited Code (LIC) assignment with Austria Prolotus Technologies Pty Ltd HIBCC*. By doing so, they afreeze GmbH Red Milawa Pty Ltd dba Magic Mobility have demonstrated their AMI GmbH SDI Limited commitment to patient safety Bender Medsystems GmbH Signostics Ltd. and logistical efficiency for BHS Technologies GmbH Sirtex Medical Pty Ltd their customers, the industry Metasys Medizintechnik GmbH Smith & Nephew Surgical Pty. Ltd. and the public at large. PAA Laboratories GmbH Staminalift International Limited Safersonic Medizinprodukte Handels The Pipette Company Pty. Ltd. Any organization that is GmbH Thermo Electron Corporation interested in using the HIBC W & H Dentalwerk Burmoos GmbH Vush Pty Ltd uniform labeling system may apply for the assignment of VUSH STIMULATION Australia one or more LICs. William A Cook Australia Pty. Ltd. Adv. Surgical Design & Manufacture, Ltd. Last updated 9-21-2021 AirPhysio Pty Ltd Belgium Annalise-AI Pty Ltd 3M Europe Apollo Medical Imaging Technology Pty Advanced Medical Diagnostics SA/NV Ltd Analis SA/NV Benra Pty Ltd dba Gelflex Laboratories Baxter World Trade Bioclone Australia Pty. Ltd. Bio-Rad RSL Candelis, Inc. Bio-Rad Lab Inc Clinical Diag. Group DePuy Australia Pty. Ltd. Biosource Europe SA For more information, please dorsaVi Ltd Cilag NV contact the HIBCC office at: EC Certification Service GmbH Coris Bioconcept Fink Engineering Pty Ltd Fuji Hunt Photographic Chemicals NV 2525 E. -

Overview of Ftc Antitrust Actions in Pharmaceutical Services and Products
OVERVIEW OF FTC ANTITRUST ACTIONS IN PHARMACEUTICAL SERVICES AND PRODUCTS Health Care Division Bureau of Competition Federal Trade Commission Washington D.C. 20580 Markus H. Meier Assistant Director Bradley S. Albert Deputy Assistant Director Saralisa C. Brau Deputy Assistant Director September 2009 TABLE OF CONTENTS Page I. INTRODUCTION. ........................................................... 1 II. CONDUCT INVOLVING PHARMACEUTICAL SERVICES AND PRODUCTS. 3 A. Monopolization. ...................................................... 3 B. Agreements Not to Compete. ............................................ 8 C. Agreements on Price or Price-Related Terms. 14 D. Agreements to Obstruct Innovative Forms of Health Care Delivery or Financing. 20 E. Illegal Tying and Other Arrangements. .................................... 20 III. PHARMACEUTICAL MERGERS. ........................................... 20 A. Horizontal Mergers Between Direct Competitors. 20 B. Potential Competition Mergers. ......................................... 44 C. Innovation Market Mergers. ............................................ 47 D. Vertical Mergers...................................................... 49 IV. INDUSTRY GUIDANCE STATEMENTS...................................... 50 A. Advisory Opinions. ................................................... 50 B. Citizen Petition to the Food and Drug Administration. 51 V. AMICUS BRIEFS. ......................................................... 51 VI. INDICES. ............................................................ -

Youth Access Tobacco Enforcement Program Annual Report 04-05
Youth Access Tobacco Enforcement Program Annual Report October 1, 2004 - September 30, 2005 � TobaccoSales To Youth New York State Department of Health Questions or requests for additional copies of this report: New York State Department of Health Bureau of Community Environmental Health & Food Protection Tobacco Enforcement Program Flanigan Square, Room 515 547 River Street Troy, NY 12180-2216 Telephone: (518) 402-7600 or 1 (800) 458-1158, ext. 27600 Fax: (518) 402-7609 This annual report of the New York State Department of Health (NYS DOH) Youth Access Tobacco Enforcement Program is prepared in accordance with Section 1399-kk of the Public Health law and is submitted by the Commissioner of Health to the Governor and the Legislature. ACKNOWLEDGEMENTS Special thanks go to the local health department enforcement officers, the New York City Department of Consumer Affairs and the youth who participated in the access compliance check surveillance initiative. Staff of the New York State Department of Health’s Bureau of Community Environmental Health and Food Protection Tobacco Enforcement Program prepared this report with data provided from the local enforcement officers, other State agencies and programs within the Department of Health. The New York State Department of Health’s Tobacco Control Program and the New York State Education Department supplied information regarding tobacco use and trends among minors. The State Department of Taxation and Finance provided registration and revenue data. The Department of State’s Office of Fire Prevention -

Long Island University Arnold & Marie Schwartz College of Pharmacy And
Long Island University Arnold & Marie Schwartz College of Pharmacy and Health Sciences 1998-2000 Undergraduate & Graduate Bulletin Arnold & Marie Schwartz College of Pharmacy and Health Sciences Long Island University 75 DeKalb Avenue, Brooklyn, NY 11201-5497 General Information: (718) 488-1000 http://www.liu.edu Admissions: (718) 488-1011 Fax: (718) 797-2399 Email: [email protected] The Arnold & Marie Schwartz College of Pharmacy and Health Sciences Undergraduate & Graduate Bulletin is issued biennially. A schedule of classes is published by the Office of the Registrar for the Fall, Spring and Summer sessions. Notice to Students. Long Island University reserves the right to delete any course described in this publication for any reason and cannot guarantee enrollment into any specific sections of courses. The University also reserves the right to effect any other changes in the curriculum, administration, tuition and fees, program offerings, or any other phase of school activity without notice. The University expects each student to have a knowledge of the information presented in the bulletin and other official publications of the various faculties and campuses pertaining to his/her course of study. For further information or specific degree requirements, prospective students should call the Admissions Office and enrolled students should speak with their advisers. College of Pharmacy and Health Sciences College of Pharmacy and Health Sciences; the Friends LONG ISLAND UNIVERSITY World Program of global education for social change; var - sity and intramural teams in 76 sports; WPBX-FM and WCWP-FM, Long Island’s Public Radio Network; the Long Island University, the United States’ eighth- world-renowned Tilles Center for the Performing Arts; largest private university measured by enrollment, is a and a proud, accomplished group of more than 135,000 multi-campus, doctoral degree-granting institution serving alumni and alumnae. -
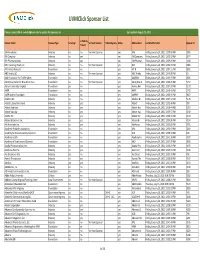
Sponsor List
UVMClick Sponsor List Please contact SPA at [email protected] to update the Sponsor List last updated August 24, 2021 Is Publicly Sponsor Name Sponsor Type Is Foreign Vermont Sponsor Federal Agency Active Abbreviation Last Modified Date Sponsor ID Traded 106 Associates Industry no no Vermont Sponsor yes 106 Friday, January 29, 2021 12:35:03 PM 3385 3M Company Industry no yes yes 3M Company Friday, January 29, 2021 12:32:32 PM 2877 3M Pharmaceuticals Industry no yes yes 3M Pharmac Friday, January 29, 2021 12:39:23 PM 4188 835 Hinesburg Road, LLC Industry no no Vermont Sponsor yes 835 Friday, January 29, 2021 12:32:33 PM 3386 A Territory Resource Foundation no no yes A.T.R. Friday, January 29, 2021 12:34:11 PM 3805 A&C Realty LLC. Industry no no Vermont Sponsor yes A&C Realty Friday, January 29, 2021 12:40:28 PM 60 AAA Foundation for Traffic Safety Foundation no no yes AAAFDN Friday, January 29, 2021 12:36:49 PM 3806 AALV/New Farms for New Americans Foundation no no Vermont Sponsor yes AALV/New FFriday, January 29, 2021 12:32:25 PM 5154 Aarhus University Hospital Foundation yes no yes Aarhus Uni Friday, January 29, 2021 12:34:23 PM 5172 AARP Foundation no no yes AARP Friday, January 29, 2021 12:40:40 PM 2742 AARP Andrus Foundation Foundation no no yes AARPAF Friday, January 29, 2021 12:36:35 PM 3807 Abalone Bio Industry no no yes Abalone Bi Friday, January 29, 2021 12:37:14 PM 5178 Abbott Laboratories Fund Industry no yes yes Abbott Friday, January 29, 2021 12:32:46 PM 309 Abbott Nutrition Industry no yes yes Abbott Nut Friday, January 29, 2021 12:38:44 PM 5219 Abbott Vascular Industry no yes yes Abbott Vas Friday, January 29, 2021 12:35:57 PM 4192 AbbVie Inc. -

Board of Director Candidates
2021 Annual General Meeting Board of Director Candidates James I. Healy, M.D., Ph.D. (Current Board Member) James I. Healy, M.D., Ph.D. has served as a member of our board of directors since November 2014. Dr. Healy has been a General Partner of Sofinnova Investments, Inc. (formerly Sofinnova Ventures), a venture capital firm, since June 2000. Prior to June 2000, Dr. Healy held various positions at Sanderling Ventures, Bayer Healthcare Pharmaceuticals (as successor to Miles Laboratories) and ISTA Pharmaceuticals, Inc. Dr. Healy is currently on the board of directors of Bolt Therapeutics, Inc. (Nasdaq: BOLT), Coherus BioSciences, Inc. (Nasdaq: CHRS), Karuna Therapeutics, Inc. (Nasdaq: KRTX), Natera, Inc. (Nasdaq: NTRA), NuCana plc (Nasdaq: NCNA), ObsEva SA (Nasdaq: OBSV) and Y-mAbs Therapeutics, Inc. (Nasdaq: YMAB) and two private companies. Previously, he served as a board member of Amarin Corporation, Auris Medical Holding AG, Edge Therapeutics, Inc., Hyperion Therapeutics, Inc., InterMune, Inc., Iterum Therapeutics PLC, Anthera Pharmaceuticals, Inc., Durata Therapeutics, Inc., CoTherix, Inc., Movetis NV and several private companies. Dr. Healy holds an M.D. and a Ph.D. in Immunology from Stanford University School of Medicine and holds a B.A. in Molecular Biology and a B.A. in Scandinavian Studies from the University of California, Berkeley. Jan Møller Mikkelsen (Current Board Member) Jan Møller Mikkelsen founded Ascendis Pharma and has served as President and Chief Executive Officer as well as Board member since December 2007 and currently serves on the board of Visen. From 2002 to 2006, Mr. Mikkelsen served as President and Chief Executive Officer of LifeCycle Pharma A/S, now Veloxis Pharmaceuticals A/S, which was a publicly traded biotechnology company. -

Brief of Consumers Union of United States, Inc., As Amicus Curiae in Support of Petitioners, Riegel & Riegel V
Georgetown University Law Center Scholarship @ GEORGETOWN LAW 2007 Brief of Consumers Union of United States, Inc., as Amicus Curiae in Support of Petitioners, Riegel & Riegel v. Medtronic, Inc., No. 06-179 (U.S. Aug. 27, 2007) Lisa Heinzerling Georgetown University Law Center Docket No. 06-179 This paper can be downloaded free of charge from: http://scholarship.law.georgetown.edu/scb/40 This open-access article is brought to you by the Georgetown Law Library. Posted with permission of the author. Follow this and additional works at: http://scholarship.law.georgetown.edu/scb Part of the Consumer Protection Law Commons, and the Torts Commons No. 06-179 ~ ~!~! GLE~a~K IN THE £ upreme ¢£ourt of nite tate CHARLES R. RIEGEL AND DONNA S. RIEGEL, Petitioners V. MEDTRONIC, INC., Respondent ON WRIT OF CERTIORARI TO THE UNITED STATES COURT OF APPEALS FOR THE SECOND CIRCUIT BRIEF OF CONSUMERS UNION OF UNITED STATES, INC., AS AMICUS CURIAE IN SUPPORT OF PETITIONERS LISA HEINZERLING Counsel of Record 600 New Jersey Ave., N.W. Washington, D.C. 20001 (202) 662-9115 MARK SAVAGE Consumers Union of United States, Inc. 1535 Mission Street San Francisco, CA 94103-2512 Counsel for Amicus Consumers Union of United States, Inc. WILSON-EPES PRINTINGCO., INC. - (202) 789-0096 - WASHINGTON, D. C. 20002 QUESTION PRESENTED Whether the express preemption provision of the Medical Device Amendments to the Food, Drug, and Cosmetic Act, 21 U.S.C. § 360k(a), preempts state-law claims seeking damages for injuries caused by medical devices that received premarket approval from the Food and Drug Administration.