Thaumetopoea Pityocampa) in Bulgaria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Main Characteristic Features of the Bulgarian Orlitza Dialect (From the East Rhodopes)
US-China Foreign Language, June 2015, Vol. 13, No. 6, 417-422 doi:10.17265/1539-8080/2015.06.003 D DAVID PUBLISHING The Main Characteristic Features of the Bulgarian Orlitza Dialect (From the East Rhodopes) Ivan Georgiev Iliev Plovdiv University, Plovdiv, Bulgaria This article describes the main characteristic features of the dialect spoken by Muslim Bulgarians inhabiting the East Rhodopian Bulgarian village of Orlitza. The dialect features phonetic changes © Æ a, ú Æ a, ќ Æ ’a, ü Æ ’a; the traces from the Old Bulgarian nasal vowels; the use of a definite article from the Old Bulgarian demonstrative pronoun òú “this” unlike most of the other Rhodopian dialects where a triple article derived from the demonstrative pronouns òú, ñü, îíú is in use; as well as interesting lexemes, often from Turkish origin. Кeywords: Bulgarian language, Bulgarian dialectology, East Rhodopes Introduction The village of Orlitza, within the municipality of Kirkovo, is situated near the Greek-Bulgarian border, not far from the Makaza pass (and the border check point with the same name). The inhabitants of the village are Muslim Bulgarians (Pomaks).1 Their dialect is almost unknown. In his book on the Tihomir dialect, Kabasanov (1963) mentions in passing several basic characteristic features of the dialectal varieties spoken in the villages of Lozengradtzi, Strizhba, Tzarinovo, and Orlitza (called Protogerovo at that time): the transition © Æ а, the traces of nasal vowels, the rare change o Æ a, the prefixal change пир- Æ при-. So far, only short articles concerning the examined here dialect of Orlitza have been published in local newspapers (Kostovska, 1971; Avdzhiev, 1988). -

Report by Institute of Viticulture and Enology, Pleven
REPORT BY INSTITUTE OF VITICULTURE AND ENOLOGY, PLEVEN BY ACTIVITY 3.2.1 .: DESCRIPTION OF WINE GRAPE VARIETIES AND MICRO AREAS OF PRODUCTION IN THE HASKOVO AND KARDZHALI DISTRICTS OCTOBER, 2018 This report was prepared by a team of scientists from the Institute of Viticulture and Enology, Pleven, Bulgaria for the purpose of the project DIONYSOS. The analysis of the report uses own research; references to scientific literature in the field of viticulture, wine, history, geography, soil science, climate and tourism of bulgarian and world scientists; official statistics of NSI, MAFF, NIMH; officially published documents such as districts and municipalies development strategies in the districts of Haskovo and Kardzhali; the Law on Wine and Spirits of the Republic of Bulgaria; the Low of Tourism of the Republic of Bulgaria; official wine cellar websites, tourist information centers, travel agencies; and other sources. This document is created under the project “Developing identity on yield, soil and site”/DIONYSOS, Subsidy contract B2.6c.04/01.11.2017 with the financial support of Cooperation Programme “Interreg V-A Greece-Bulgaria” 2014-2020, Co- funded by the European Regional Development Fund and National funds of Greece and Bulgaria. The entire responsibility for the contents of the document rests with Institute of Viticulture and Enology-Pleven and under no circumstances it can be assumed that the materials and information on the document reflects the official view European Union and the Managing Authority Този документ е създаден в рамките на проект „Разработване на идентичност на добива, почвите и местностите“/ДИОНИСОС, Договор за субсидиране B2.6c.04/01.11.2017 който се осъществява с финансовата подкрепа на подкрепа на Програма за трансгранично сътрудничество ИНТЕРРЕГ V-A Гърция-България 2014-2020, съфинансирана от Европейския фонд за регионално развитие и от националните фондове на страните Гърция и България. -

България Bulgaria Държава Darzhava
Проект "Разбираема България" Вид Транслитера Собствено име Транслитерация на собственото име Вид обект Транслитерация Местоположение Транслитерация местоположение ция България Bulgaria държава darzhava Абаджиев Abadzhiev фамилно име familno ime Абаджийска Abadzhiyska улица ulitsa Сливен Sliven град grad Абаносов Abanosov фамилно име familno ime Абдовица Abdovitsa квартал kvartal София Sofia град grad Абланица Ablanitsa село selo Абланов Ablanov фамилно име familno ime Абланово Ablanovo улица ulitsa Сливен Sliven град grad Абланово Ablanovo улица ulitsa Ямбол Yambol град grad Аблановска низина Ablanovska nizina низина nizina Абоба Aboba улица ulitsa Бургас Burgas град grad Абоба Aboba улица ulitsa Разград Razgrad град grad Абоба Aboba улица ulitsa София Sofia град grad Абрашев Abrashev фамилно име familno ime Абрашков Abrashkov фамилно име familno ime Абрит Abrit село selo Абритус Abritus улица ulitsa Разград Razgrad град grad Ав. Гачев Av. Gachev улица ulitsa Габрово Gabrovo град grad Ав. Митев Av. Mitev улица ulitsa Враца Vratsa град grad Ав. Стоянов Av. Stoyanov улица ulitsa Варна Varna град grad Аваков Avakov фамилно име familno ime Авгостин Avgostin лично име lichno ime Август Avgust лично име lichno ime Август Попов Avgust Popov улица ulitsa Шумен Shumen град grad Августа Avgusta лично име lichno ime Августин Avgustin лично име lichno ime Августина Avgustina лично име lichno ime Авджиев Avdzhiev фамилно име familno ime Аверкий Averkiy улица ulitsa Кюстендил Kyustendil град grad Авксентий Велешки Avksentiy Veleshki улица ulitsa Варна -

Annexes to Rural Development Programme
ANNEXES TO RURAL DEVELOPMENT PROGRAMME (2007-2013) TABLE OF CONTENTS Annex 1 ...........................................................................................................................................4 Information on the Consultation Process ........................................................................................4 Annex 2 .........................................................................................................................................13 Organisations and Institutions Invited to the Monitoring Committee of the Implementation of the Rural Development Programme 2007-2013 .................................................................................13 Annex 3 .........................................................................................................................................16 Baseline, Output, Result and Impact Indicators............................................................................16 Annex 4 .........................................................................................................................................29 Annexes to the Axis 1 Measures...................................................................................................29 Attachment 1 (Measure 121 Modernisation of Agricultural Holding) .........................................30 List of Newly Introduced Community Standards .........................................................................30 Attachment 1.A. (Measure 121 Modernisation of Agricultural Holding -
List of Appendices
Preparation of Regional Water and Wastewater Master Plans for Central Region Regional Final Master Plan for ViK OOD - Kardzhali LIST OF APPENDICES APPENDIX 1-1 PROJECTS UNDER IMPLEMENTATION ............................................................................................. 1 APPENDIX 1-2 REGULATORY FRAMEWORK IN BULGARIA...................................................................................... 3 APPENDIX 1-3 REGULATORY FRAMEWORK IN THE EUROPEAN COMMUNITY ....................................................... 9 APPENDIX 2-1 PROJECT SCOPE ............................................................................................................................. 12 APPENDIX 2-2 ADMINISTRATIVE DATA ................................................................................................................ 13 APPENDIX 2-3 MAPS OF GROUNDWATER BODIES ................................................................................................ 22 APPENDIX 2-4 DRINKING WATER MONITORING FOR THE YEAR 2012 ................................................................... 26 APPENDIX 3-1 GENERAL FEATURES OF SURFACE WATER...................................................................................... 28 APPENDIX 3-2 GROUNDWATER QUANTITY .......................................................................................................... 32 APPENDIX 3-3 WATER SUPPLY ZONES ................................................................................................................. 39 APPENDIX 3-4 GROUNDWATER -
Област Кърджали, България District Kardzhali, Bulgaria
ОБЛАСТ КЪРДЖАЛИ, БЪЛГАРИЯ Област Кърджали заема 3 216 км2 площ в югоизточната част на Република България, което представлява 2.9% от територията на България. На запад граничи със Смолянска област, на север с Хасковска област, на юг и югоизток с Република Гърция. Заема северните склонове на Източните Родопи. Обхваща 7 общини - Ардино, Джебел, Кирково, Крумовград, Кърджали, Момчилград и Черноочене. Мекият климат, благоприятното географско разположение, богатото културно-историческо наследство, двете “водни езера” - язовирите пре- доставят възможност за целогодишно развитие на всички форми на туризма. Голямото биоразнообразие, уникалните природни забележителности и запазена природна среда, незасегната от негативните влияния на индустриализацията и урбанизацията превръщат еко-туризъм в един от приоритетите за развитие на общината. На територията й има няколко защитени природни обекта - природните забележителности “Каменна сватба”; “Каменни гъби” край с.Бели пласт; “Скален прозорец” край с.Костино, а също и няколко защитени местности с находища на световно застрашени представители на флората и фауната - “Венерин косъм”, “Юмрук скала” и “Средна Арда”. DISTRICT KARDZHALI, BULGARIA District Kardzhali covers an area of 3 216 sq. klm. in South-Eastern part of Repub- lic of Bulgaria, which represents 2.9% of territory of Bulgaria. On West side it bor- ders with Smolian District, North side with Haskovo district and South and South West side with Republic of Greece. It covers the slopes of Eastern Rhodopes. 7 municipalities are there - Ardino, Djebel, Kirkovo, Krumovgrad, Kardzhali, Mom- chilgrad and Chernoochene. The mild climate, favorable geographic situation, rich culture-historical heritage, the two ”water lakes” - the artificial water lakes give possibility for round of year development of all forms of tourism. -

Pagina 1 Di 17 13/10/2014
Pagina 1 di 17 Print Bluetongue, Bulgaria Close Information received on 24/09/2014 from Pr. Nikola T. Belev, OIE Delegate, OIE Regional Representation for Eastern Europe, Ministry of Agriculture and Food , SOFIA, Bulgaria Summary Report type Follow-up report No. 5 Date of start of the event 04/07/2014 Date of pre-confirmation of the 04/07/2014 event Report date 24/09/2014 Date submitted to OIE 24/09/2014 Reason for notification Reoccurrence of a listed disease Date of previous occurrence 27/03/2008 Manifestation of disease Clinical disease Causal agent Bluetongue virus Serotype 4 Nature of diagnosis Clinical, Laboratory (basic) This event pertains to the whole country Immediate notification (15/07/2014) Follow-up report No. 1 (07/08/2014) Follow-up report No. 2 (08/08/2014) Related reports Follow-up report No. 3 (01/09/2014) Follow-up report No. 4 (08/09/2014) Follow-up report No. 5 (24/09/2014) New outbreaks (119) Outbreak 1 Velika, Tsarevo, Burgas, BURGAS Date of start of the outbreak 29/07/2014 Outbreak status Continuing (or date resolved not provided) Epidemiological unit Farm Species Susceptible Cases Deaths Destroyed Slaughtered Affected animals Goats 50 0 0 0 0 Sheep 37 2 1 0 0 Outbreak 2 Kisiitsite, Tryavna, Gabrovo, GABROVO Date of start of the outbreak 30/07/2014 Outbreak status Continuing (or date resolved not provided) Epidemiological unit Farm Species Susceptible Cases Deaths Destroyed Slaughtered Affected animals Sheep 11 1 1 0 0 Outbreak 3 Belitsa, Tryavna, Gabrovo, GABROVO Date of start of the outbreak 31/07/2014 Outbreak -

Annexes to Rural Development Programme
ANNEXES TO RURAL DEVELOPMENT PROGRAMME (2007-2013) TABLE OF CONTENTS Annex 1 ...........................................................................................................................................4 Information on the Consultation Process ........................................................................................4 Annex 2 .........................................................................................................................................13 Organisations and Institutions Invited to the Monitoring Committee of the Implementation of the Rural Development Programme 2007-2013 .................................................................................13 Annex 3 .........................................................................................................................................16 Baseline, Output, Result and Impact Indicators............................................................................16 Annex 4 .........................................................................................................................................29 Annexes to the Axis 1 Measures...................................................................................................29 Attachment 1 (Measure 121 Modernisation of Agricultural Holding) .........................................30 List of Newly Introduced Community Standards .........................................................................30 Attachment 2 (Measure 123 Adding value of agricultural and forestry -
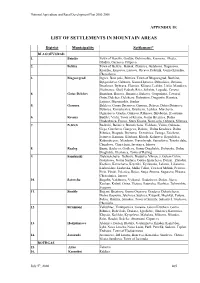
List of Settlements in Mountain Areas
National Agriculture and Rural Development Plan 2000-2006 APPENDIX 1E LIST OF SETTLEMENTS IN MOUNTAIN AREAS District Municipality Settlement* BLAGOEVGRAD 1. Bansko Town of Bansko, Gostun, Dobrinishte, Kremena, Mesta, Obidim, Osenovo, Filipovo 2. Belitza Town of Belitza , Babiak, Zlatarica, Gylybovo, Dagonovo, Kraishte, Kuzaovo, Liutovo, Orcevo, Palatnik, Gorno Kraishte, Chereshovo 3. Blagoevgrad Izgrev, Belo pole, Bistrica, Town of Blagoevgrad, Buchino, Bylgarchevo, Gabrovo, Gorno Hyrsovo, Debochica, Delvino, Drenkovo, Dybrava, Elenovo, Klisura, Leshko, Lisiia, Marulevo, Moshtanec, Obel, Padesh, Rilci, Selishte, Logodaj, Cerovo 4. Gotze Delchev Banichan, Borovo, Breznica, Bukovo, Gospodinci, Town of Gotze Delchev, Delchevo, Dobrotino, Dragostin, Kornica, Lyjnica, Musomishta, Sredna 5. Garmen Baldevo, Gorno Drianovo, Garmen, Debren, Dolno Drianovo, Dybnica, Kovachevica, Krushevo, Leshten, Marchevo, Ognianovo, Oreshe, Osikovo, Ribnovo, Skrebatno, Hvostiane 6. Kresna Budilci, Vlahi, Town of Kresna, Gorna Breznica, Dolna Gradeshnica, Ezerec, Stara Kresna, Novo selo, Oshtava, Slivnica 7. Petrich Baskalci, Belasica, Borovichene, Vishlene, Volno, Gabrene, Gega, Gorchevo, Giurgevo, Dolene, Dolna Krushica, Dolna Ribnica, Dragush, Drenovo, Drenovica, Zanoga, Zoichene, Ivanovo, Kamena, Kladenci, Kliuch, Kolarovo, Kryndjilica, Kukurahcevo, Mendovo, Pravo byrdo, Samuilovo, Tonsko dabe, Churilovo, Churicheni, Iavornica, Iakovo 8. Razlog Bania, Bachevo, Godlevo, Gorno Draglishte, Dobyrsko, Dolno Draglishte, Eleshnica, Town of Razlog 9. Sandanski Belevehchevo, -

Lütvi Mestan
“МАРС 55” ООД П р о д а ж б а н а а п а р т а м е н т и , гаражи и търговски обекти GSM: 0884067245 GSM: 0884067246 BULGARİSTAN TÜRKLERİNİN SESİ 24 KırcaaliNisan 2013 Çarşamba - Yıl:VII Sayı:16 (180) Fiyatı: 0,50 Haber lv. www.kircaalihaber.com İSSN 1313-6925 Türk Sanatçılar Kırcaalilerle Buluştu Resmiye MÜMÜN vatandaşlar, geleneksel 25 öğretmenle buluş- Türk tiyatrosunun med- ması gerçekleşti. Biri Edirne Valiliği’nin Sa- dah, Hacivat-Karagöz şiir, diğeri Hilmi Yavuz’la natçılar Balkanlarla gölge oyunu ustası yapılan söyleşileri içe- Buluşuyor projesine Seçkin Bayramoğlu'nun ren iki kitabını Türkçe Kırcaali’de Ömer Lütfi sunduğu gösteriyi bü- sevdalılarına hediye etti. Kültür Derneği’nde start yük beğeniyle izlediler. Sayın Ercan Yılmaz, her verildi. Derneğin kütüp- Sayın Fahri Tuna, bir öğretmenle ayrı ayrı hanesinde Edirne Vali- Ömer Lütfi Kültür tanışıp sohbet ederek liği Balkan Danışmanı, Derneği’ne bağlı Re- Türkçenin, dünü, bugü- yazar, Balkan Türküsü cep Küpçü Edebiyat nü ve geleceği üzerine Dergisi Genel Yayın Kulübü üyesi yaklaşık fikir alışverişinde bulun- Yönetmeni Fahri Tuna 15 şair ve yazarla hem du. Doğu Rodoplar’dan Balkan Türküsü dergi- Daha sonra ilk defa şair, yazar ve ressam- si, hem ortaklaşa nasıl Kırcaali’de meddah, larla görüşürken, diğer edebiyat etkinlikleri ger- orta oyunu ve Karagöz- salonda şair Ercan Yıl- çekleştirebilinir üzerine Hacivat gösterisi sunul- maz bölgeden Türkçe güzel bir sohbet ede- du. Kırcaaliler, meddah, öğretmenleriyle görüş- rek görüş alışverişinde Karagöz-Hacivat gölge projelerden biri Doğu yayınladığı dergilerden limler Lisesi’nde Türk tü. Daha sonra Kırcaalili bulundu. İleriye yönelik oyunu ustası Seçkin Rodoplar’dan şair ve ya- başka Akşamın Aydın- Dili ve Edebiyatı öğret- Bayramoğlu'nun saye- zarların eserlerini içeren lığında Portreler başlıklı meni, şair Ercan Yıl- sinde doyasıya eğlen- SEFATERMAL-GAZLIGÖL bir antolojinin basılması.