EU Comment the EU Would Like to Commend the OIE Aquatic Animal Health Standards Commission for Its Work and for Having Taken
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Disease List for Aquaculture Health Certificate
Quarantine Standard for Designated Species of Imported/Exported Aquatic Animals [Attached Table] 4. Listed Diseases & Quarantine Standard for Designated Species Listed disease designated species standard Common name Disease Pathogen 1. Epizootic haematopoietic Epizootic Perca fluviatilis Redfin perch necrosis(EHN) haematopoietic Oncorhynchus mykiss Rainbow trout necrosis virus(EHNV) Macquaria australasica Macquarie perch Bidyanus bidyanus Silver perch Gambusia affinis Mosquito fish Galaxias olidus Mountain galaxias Negative Maccullochella peelii Murray cod Salmo salar Atlantic salmon Ameirus melas Black bullhead Esox lucius Pike 2. Spring viraemia of Spring viraemia of Cyprinus carpio Common carp carp, (SVC) carp virus(SVCV) Grass carp, Ctenopharyngodon idella white amur Hypophthalmichthys molitrix Silver carp Hypophthalmichthys nobilis Bighead carp Carassius carassius Crucian carp Carassius auratus Goldfish Tinca tinca Tench Sheatfish, Silurus glanis European catfish, wels Negative Leuciscus idus Orfe Rutilus rutilus Roach Danio rerio Zebrafish Esox lucius Northern pike Poecilia reticulata Guppy Lepomis gibbosus Pumpkinseed Oncorhynchus mykiss Rainbow trout Abramis brama Freshwater bream Notemigonus cysoleucas Golden shiner 3.Viral haemorrhagic Viral haemorrhagic Oncorhynchus spp. Pacific salmon septicaemia(VHS) septicaemia Oncorhynchus mykiss Rainbow trout virus(VHSV) Gadus macrocephalus Pacific cod Aulorhynchus flavidus Tubesnout Cymatogaster aggregata Shiner perch Ammodytes hexapterus Pacific sandlance Merluccius productus Pacific -

(Sea of Okhotsk, Sakhalin Island): 2. Cyclopteridae−Molidae Families
ISSN 0032-9452, Journal of Ichthyology, 2018, Vol. 58, No. 5, pp. 633–661. © Pleiades Publishing, Ltd., 2018. An Annotated List of the Marine and Brackish-Water Ichthyofauna of Aniva Bay (Sea of Okhotsk, Sakhalin Island): 2. Cyclopteridae−Molidae Families Yu. V. Dyldina, *, A. M. Orlova, b, c, d, A. Ya. Velikanove, S. S. Makeevf, V. I. Romanova, and L. Hanel’g aTomsk State University (TSU), Tomsk, Russia bRussian Federal Research Institute of Fishery and Oceanography (VNIRO), Moscow, Russia cInstitute of Ecology and Evolution, Russian Academy of Sciences (IPEE), Moscow, Russia d Dagestan State University (DSU), Makhachkala, Russia eSakhalin Research Institute of Fisheries and Oceanography (SakhNIRO), Yuzhno-Sakhalinsk, Russia fSakhalin Basin Administration for Fisheries and Conservation of Aquatic Biological Resources—Sakhalinrybvod, Aniva, Yuzhno-Sakhalinsk, Russia gCharles University in Prague, Prague, Czech Republic *e-mail: [email protected] Received March 1, 2018 Abstract—The second, final part of the work contains a continuation of the annotated list of fish species found in the marine and brackish waters of Aniva Bay (southern part of the Sea of Okhotsk, southern part of Sakhalin Island): 137 species belonging to three orders (Perciformes, Pleuronectiformes, Tetraodon- tiformes), 31 family, and 124 genera. The general characteristics of ichthyofauna and a review of the commer- cial fishery of the bay fish, as well as the final systematic essay, are presented. Keywords: ichthyofauna, annotated list, conservation status, commercial importance, marine and brackish waters, Aniva Bay, southern part of the Sea of Okhotsk, Sakhalin Island DOI: 10.1134/S0032945218050053 INTRODUCTION ANNOTATED LIST OF FISHES OF ANIVA BAY The second part concludes the publication on the 19. -

Pleuronectidae
FAMILY Pleuronectidae Rafinesque, 1815 - righteye flounders [=Heterosomes, Pleronetti, Pleuronectia, Diplochiria, Poissons plats, Leptosomata, Diprosopa, Asymmetrici, Platessoideae, Hippoglossoidinae, Psettichthyini, Isopsettini] Notes: Hétérosomes Duméril, 1805:132 [ref. 1151] (family) ? Pleuronectes [latinized to Heterosomi by Jarocki 1822:133, 284 [ref. 4984]; no stem of the type genus, not available, Article 11.7.1.1] Pleronetti Rafinesque, 1810b:14 [ref. 3595] (ordine) ? Pleuronectes [published not in latinized form before 1900; not available, Article 11.7.2] Pleuronectia Rafinesque, 1815:83 [ref. 3584] (family) Pleuronectes [senior objective synonym of Platessoideae Richardson, 1836; family name sometimes seen as Pleuronectiidae] Diplochiria Rafinesque, 1815:83 [ref. 3584] (subfamily) ? Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Poissons plats Cuvier, 1816:218 [ref. 993] (family) Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Leptosomata Goldfuss, 1820:VIII, 72 [ref. 1829] (family) ? Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Diprosopa Latreille, 1825:126 [ref. 31889] (family) Platessa [no stem of the type genus, not available, Article 11.7.1.1] Asymmetrici Minding, 1832:VI, 89 [ref. 3022] (family) ? Pleuronectes [no stem of the type genus, not available, Article 11.7.1.1] Platessoideae Richardson, 1836:255 [ref. 3731] (family) Platessa [junior objective synonym of Pleuronectia Rafinesque, 1815, invalid, Article 61.3.2 Hippoglossoidinae Cooper & Chapleau, 1998:696, 706 [ref. 26711] (subfamily) Hippoglossoides Psettichthyini Cooper & Chapleau, 1998:708 [ref. 26711] (tribe) Psettichthys Isopsettini Cooper & Chapleau, 1998:709 [ref. 26711] (tribe) Isopsetta SUBFAMILY Atheresthinae Vinnikov et al., 2018 - righteye flounders GENUS Atheresthes Jordan & Gilbert, 1880 - righteye flounders [=Atheresthes Jordan [D. -

602300Pdf.Pdf
Table of Contents ORAL PRESENTATIONS ................................................................................................................. 45 A Case Study on the Pollinator Bee Diversity: Barcoding the Members of the Genus Halictus s. str. Latreille (Halictidae: Apoidea: Hymenoptera) of Turkey ........................................................... 12 A New Entomopathogen from Alticahampei (Allard, 1867) (Coleoptera: Chrysomelidae) ..................... 13 A new genus record For Turkey Spider Fauna, Floronia Simon 1887(Araneae/Linyphiidae).................. 14 A New record of rake legged mites from Turkey: Allocaeculus multispinosus (Acari: Caeculidae) ........ 15 Age structures and Growth Parameters in three populations of Levanten Frog, Pelophylax bedriagae .... 16 An Interesting Dragonfly Record, Selysiothemis nigra (Vander Linden, 1825) ...................................... 17 Aphids and Their Densities on Brassicaceae Plants in Diyarbakir Province of Turkey ........................... 18 Ascidians (Tunicata, Urochordata) Fauna of Turkey Coasts ................................................................. 19 Asymmetric variations in some species of the genus Raphignathus (Acari: Raphignathidae) ................. 20 Blood Cell Morphology and Blood Biochemistry of Pelophylax bedriagae ........................................... 21 Carabidae (Coleoptera) Records from Upland-Meadows of Türkmen Mountain (Kütahya-Eskişehir), Turkey ................................................................................................................................... -
Genetic Diversity, Population Structure and Historical Demography
www.nature.com/scientificreports OPEN Genetic diversity, population structure and historical demography of the two‑spined yellowtail stargazer (Uranoscopus cognatus) Nur Ilham Syahadah Mohd Yusof1, Tun Nurul Aimi Mat Jaafar1, Veera Vilasri2, Siti Azizah Mohd Nor3, Ying Giat Seah1,4, Ahasan Habib1,5, Li Lian Wong3, Muhd Danish‑Daniel3, Yeong Yik Sung3, Abd. Ghafar Mazlan6, Rumeaida Mat Piah1, Shahrol Idham Ismail1 & Min Pau Tan1,3* Benthic species, though ecologically important, are vulnerable to genetic loss and population size reduction due to impacts from fshing trawls. An assessment of genetic diversity and population structure is therefore needed to assist in a resource management program. To address this issue, the two‑spined yellowtail stargazer (Uranoscopus cognatus) was collected within selected locations in the Indo‑West Pacifc (IWP). The partial mitochondrial DNA cytochrome c oxidase subunit 1 and the nuclear DNA recombination activating gene 1 were sequenced. Genetic diversity analyses revealed that the populations were moderately to highly diversifed (haplotype diversity, H = 0.490–0.900, nucleotide diversity, π = 0.0010–0.0034) except sampling station (ST) 1 and 14. The low diversity level, however was apparent only in the matrilineal marker (H = 0.118–0.216; π = 0.0004–0.0008), possibly due to stochastic factors or anthropogenic stressors. Population structure analyses revealed a retention of ancestral polymorphism that was likely due to incomplete lineage sorting in U. cognatus, and prolonged vicariance by the Indo‑Pacifc Barrier has partitioned them into separate stock units. Population segregation was also shown by the phenotypic divergence in allopatric populations, regarding the premaxillary protrusion, which is possibly associated with the mechanism for upper jaw movement in biomechanical feeding approaches. -

Some Biological Aspects of Atlantic Stargazer Uranoscopus Scaber Linnaeus, 1758 (Family: Uranoscopidae) in the Egyptian Mediterranean
Turkish Journal of Fisheries and Aquatic Sciences 9: 59-66 (2009) Some Biological Aspects of Atlantic Stargazer Uranoscopus scaber Linnaeus, 1758 (Family: Uranoscopidae) in The Egyptian Mediterranean Water Samir I. Rizkalla1, Shnoudy A. Bakhoum1,* 1 National Institute of Oceanography and Fisheries, Alexandria, Egypt. * Corresponding Author: Tel.: +203.4807138; Fax: +203.4801174; Received 23 June 2008 E-mail: [email protected] Accepted 05 January 2009 Abstract Atlantic stargazer (Uranoscopus scaber Linnaeus, 1758) fish samples were monthly collected during 2001-2002 from landing of bottom trawlers operating off Alexandria, Egypt. The results revealed that this species did not exceed five years of age and females were larger than males, reaching 30.0 cm and 26.0 cm, respectively. The study of length-weight relationship showed that no significant difference between males and females (P>0.05). Growth performance in length (Ф/) and weight (Ф) were found to be 2.62 and 1.05, respectively. The von Bertalanffy growth parameters were found to be L∞ =35.02 cm, K=0.3424 yr-1 and to=- 1.011 yr. The rates of total mortality (Z), natural mortality (M) and fishing mortality (F) were 0.901 -1 -1 -1 yr , 0.554 yr and 0.347 yr , respectively. This population is characterized by low exploitation rate (E= 0.229). Keywords: age, growth, mortality, Uranoscopus scaber, South Eastern Mediterranean. Introduction collected on a monthly basis during 2001-2002 from the commercial catches of trawlers landed at The Atlantic stargazer (Uranoscopus scaber Alexandria port, Egypt. For each fish, total length Linnaeus, 1758) is a benthic species occurring in the (TL) was measured to the nearest cm, total and gutted littoral waters and on the continental shelf and upper weight to the nearest 0.01 g and sex was recorded. -

Marine Fishes from Galicia (NW Spain): an Updated Checklist
1 2 Marine fishes from Galicia (NW Spain): an updated checklist 3 4 5 RAFAEL BAÑON1, DAVID VILLEGAS-RÍOS2, ALBERTO SERRANO3, 6 GONZALO MUCIENTES2,4 & JUAN CARLOS ARRONTE3 7 8 9 10 1 Servizo de Planificación, Dirección Xeral de Recursos Mariños, Consellería de Pesca 11 e Asuntos Marítimos, Rúa do Valiño 63-65, 15703 Santiago de Compostela, Spain. E- 12 mail: [email protected] 13 2 CSIC. Instituto de Investigaciones Marinas. Eduardo Cabello 6, 36208 Vigo 14 (Pontevedra), Spain. E-mail: [email protected] (D. V-R); [email protected] 15 (G.M.). 16 3 Instituto Español de Oceanografía, C.O. de Santander, Santander, Spain. E-mail: 17 [email protected] (A.S); [email protected] (J.-C. A). 18 4Centro Tecnológico del Mar, CETMAR. Eduardo Cabello s.n., 36208. Vigo 19 (Pontevedra), Spain. 20 21 Abstract 22 23 An annotated checklist of the marine fishes from Galician waters is presented. The list 24 is based on historical literature records and new revisions. The ichthyofauna list is 25 composed by 397 species very diversified in 2 superclass, 3 class, 35 orders, 139 1 1 families and 288 genus. The order Perciformes is the most diverse one with 37 families, 2 91 genus and 135 species. Gobiidae (19 species) and Sparidae (19 species) are the 3 richest families. Biogeographically, the Lusitanian group includes 203 species (51.1%), 4 followed by 149 species of the Atlantic (37.5%), then 28 of the Boreal (7.1%), and 17 5 of the African (4.3%) groups. We have recognized 41 new records, and 3 other records 6 have been identified as doubtful. -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -

Japan Update to 05.04.2021 Approval No Name Address Products Number FROZEN CHUM SALMON DRESSED (Oncorhynchus Keta)
Japan Update to 05.04.2021 Approval No Name Address Products Number FROZEN CHUM SALMON DRESSED (Oncorhynchus keta). FROZEN DOLPHINFISH DRESSED (Coryphaena hippurus). FROZEN JAPANESE SARDINE ROUND (Sardinops 81,Misaki-Cho,Rausu- Kaneshin Tsuyama melanostictus). FROZEN ALASKA POLLACK DRESSED (Theragra chalcogramma). 1 VN01870001 Cho, Menashi- Co.,Ltd FROZEN ALASKA POLLACK ROUND (Theragra chalcogramma). FROZEN PACIFIC COD Gun,Hokkaido,Japan DRESSED. (Gadus macrocephalus). FROZEN PACIFIC COD ROUND. (Gadus macrocephalus) Maekawa Shouten Hokkaido Nemuro City Fresh Fish (Excluding Fish By-Product); Fresh Bivalve Mollusk.; Frozen Fish (Excluding 2 VN01860002 Co., Ltd Nishihamacho 10-177 Fish By-Product); Frozen Processed Bivalve Mollusk; Frozen Chum Salmon(Round,Dressed,Semi-Dressed,Fillet,Head,Bone,Skin); Frozen 1-35-1 Alaska Pollack(Round,Dressed,Semi-Dressed,Fillet); Frozen Pacific Taiyo Sangyo Co.,Ltd. 3 VN01840003 Showachuo,Kushiro- Cod(Round,Dressed,Semi-Dressed,Fillet); Frozen Pacific Saury(Round,Dressed,Semi- Kushiro Factory City,Hokkaido,Japan Dressed); Frozen Chub Mackerel(Round,Fillet); Frozen Blue Mackerel(Round,Fillet); Frozen Salted Pollack Roe 3-9 Komaba- Taiyo Sangyo Co.,Ltd. 4 VN01860004 Cho,Nemuro- Frozen Fish ; Frozen Processed Fish; (Excluding By-Product) Nemuro Factory City,Hokkaido,Japan 3-2-20 Kitahama- Marutoku Abe Suisan 5 VN01920005 Cho,Monbetu- Frozen Chum Salmon Dressed; Frozen Salmon Dressed Co.,Ltd City,Hokkaido,Japan Frozen Chum Salmon(Round,Semi-Dressed,Fillet); Frozen Salmon Milt; Frozen Pink Salmon(Round,Semi-Dressed,Dressed,Fillet); -
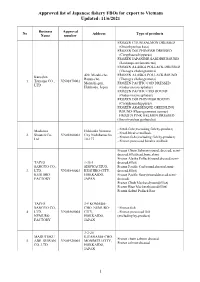
Approved List of Japanese Fishery Fbos for Export to Vietnam Updated: 11/6/2021
Approved list of Japanese fishery FBOs for export to Vietnam Updated: 11/6/2021 Business Approval No Address Type of products Name number FROZEN CHUM SALMON DRESSED (Oncorhynchus keta) FROZEN DOLPHINFISH DRESSED (Coryphaena hippurus) FROZEN JAPANESE SARDINE ROUND (Sardinops melanostictus) FROZEN ALASKA POLLACK DRESSED (Theragra chalcogramma) 420, Misaki-cho, FROZEN ALASKA POLLACK ROUND Kaneshin Rausu-cho, (Theragra chalcogramma) 1. Tsuyama CO., VN01870001 Menashi-gun, FROZEN PACIFIC COD DRESSED LTD Hokkaido, Japan (Gadus macrocephalus) FROZEN PACIFIC COD ROUND (Gadus macrocephalus) FROZEN DOLPHIN FISH ROUND (Coryphaena hippurus) FROZEN ARABESQUE GREENLING ROUND (Pleurogrammus azonus) FROZEN PINK SALMON DRESSED (Oncorhynchus gorbuscha) - Fresh fish (excluding fish by-product) Maekawa Hokkaido Nemuro - Fresh bivalve mollusk. 2. Shouten Co., VN01860002 City Nishihamacho - Frozen fish (excluding fish by-product) Ltd 10-177 - Frozen processed bivalve mollusk Frozen Chum Salmon (round, dressed, semi- dressed,fillet,head,bone,skin) Frozen Alaska Pollack(round,dressed,semi- TAIYO 1-35-1 dressed,fillet) SANGYO CO., SHOWACHUO, Frozen Pacific Cod(round,dressed,semi- 3. LTD. VN01840003 KUSHIRO-CITY, dressed,fillet) KUSHIRO HOKKAIDO, Frozen Pacific Saury(round,dressed,semi- FACTORY JAPAN dressed) Frozen Chub Mackerel(round,fillet) Frozen Blue Mackerel(round,fillet) Frozen Salted Pollack Roe TAIYO 3-9 KOMABA- SANGYO CO., CHO, NEMURO- - Frozen fish 4. LTD. VN01860004 CITY, - Frozen processed fish NEMURO HOKKAIDO, (excluding by-product) FACTORY JAPAN -

A Survey of Marine Bony Fishes of the Gaza Strip, Palestine
The Islamic University of Gaza الجــــــــــامعة اﻹســــــــــﻻميـة بغــــــــــــــــــزة Deanship of Research and Graduate Studies عمادة البحث العممي والدراسات العميا Faculty of Science كــــميـــــــــــــــــــــــــــــــة العـمـــــــــــــــــــــــــــــــــــــوم Biological Sciences Master Program ماجـســــــــتيـر العمــــــــــــــوم الحـياتيــــــــــة )Zoology) عمـــــــــــــــــــــــــــــــــــــــم حيـــــــــــــــــــــــــــــــــــــــوا A Survey of Marine Bony Fishes of the Gaza Strip, Palestine ِـخ ٌﻷؿّان اؼٌظٍّح اٌثذغٌح فً لطاع غؼج، فٍـطٍٓ By Huda E. Abu Amra B.Sc. Biology Supervised by Dr. Abdel Fattah N. Abd Rabou Associate Professor of Environmental Sciences A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Biological Science (Zoology) August, 2018 إلــــــــــــــغاع أٔا اٌّىلغ أصٔاٖ ِمضَ اٌغؿاٌح اٌتً تذًّ اؼٌٕىاْ: A Survey of Marine Bony Fishes in the Gaza Strip, Palestine ِـخ ٌﻷؿّان اؼٌظٍّح اٌثذغٌح فً لطاع غؼج، فٍـطٍٓ أقش تأٌ يا اشرًهد عهّٛ ْزِ انشعانح إًَا ْٕ َراض جٓذ٘ انخاص، تاعرصُاء يا ذًد اﻹشاسج إنّٛ حٛصًا ٔسد، ٔأٌ ْزِ انشعانح ككم أٔ أ٘ جضء يُٓا نى ٚقذو يٍ قثم اٜخشٍٚ نُٛم دسجح أٔ نقة عهًٙ أٔ تحصٙ نذٖ أ٘ يؤعغح ذعهًٛٛح أٔ تحصٛح أخشٖ. Declaration I understand the nature of plagiarism, and I am aware of the University’s policy on this. The work provided in this thesis, unless otherwise referenced, is the researcher's own work, and has not been submitted by others elsewhere for any other degree or qualification. اعى انطانثح ْذٖ عٛذ عٛذ أتٕ عًشج :Student's name انرٕقٛع Signature: Huda انراسٚخ Date: 8-8-2018 I ٔتٍجح اٌذىُ ػٍى أطغودح ِاجـتٍغ II Abstract The East Mediterranean Sea is home to a wealth of marine resources including the bony fishes (Class Osteichthyes), which constitute a capital source of protein worldwide. -

Rancangan Keputusan Menteri Kelautan Dan
RANCANGAN KEPUTUSAN MENTERI KELAUTAN DAN PERIKANAN REPUBLIK INDONESIA NOMOR /KEPMEN-KP/2018 TENTANG PENETAPAN JENIS-JENIS HAMA DAN PENYAKIT IKAN KARANTINA, GOLONGAN, DAN MEDIA PEMBAWA DENGAN RAHMAT TUHAN YANG MAHA ESA MENTERI KELAUTAN DAN PERIKANAN REPUBLIK INDONESIA, Menimbang : a. bahwa dengan semakin berkembangnya jenis-jenis hama dan penyakit ikan karantina di luar negeri, serta dalam rangka pelaksanaan pencegahan dan pengendalian penyebaran hama dan penyakit ikan karantina, perlu meninjau kembali Keputusan Menteri Kelautan dan Perikanan Nomor 80/KEPMEN-KP/2015 tentang Penetapan Jenis-Jenis Hama dan Penyakit Ikan Karantina, Golongan, Media Pembawa dan Sebarannya; b. bahwa untuk itu perlu menetapkan Keputusan Menteri Kelautan dan Perikanan tentang Penetapan Jenis-Jenis Hama dan Penyakit Ikan Karantina, Golongan, dan Media Pembawa; Mengingat : 1. Undang-Undang Nomor 16 Tahun 1992 tentang Karantina Hewan, Ikan, dan Tumbuhan (Lembaran Negara Republik Indonesia Tahun 1992 Nomor 56, Tambahan Lembaran Negara Nomor 3482); 2. Peraturan Pemerintah Nomor 15 Tahun 2002 tentang Karantina Ikan (Lembaran Negara Republik Indonesia Tahun 2002 Nomor 36, Tambahan Lembaran Negara Nomor 4197); 3. Peraturan Presiden Nomor 7 Tahun 2015 tentang Organisasi Kementerian Negara (Lembaran Negara Republik Indonesia Tahun 2015 Nomor 8); 4. Peraturan Presiden Nomor Nomor 63 Tahun 2015 tentang Kementerian Kelautan dan Perikanan (Lembaran Negara Republik Indonesia Tahun 2015 Nomor 111), sebagaimana telah diubah dengan Peraturan Presiden Nomor 2 Tahun 2017 tentang