Comparative Efficacy and Safety of Antipsychotic Drugs for Tic Disorders: a Systematic Review and Bayesian Network Meta-Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antipsychotics and Seizures: Higher Risk with Atypicals?
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Seizure 22 (2013) 141–143 Contents lists available at SciVerse ScienceDirect Seizure jou rnal homepage: www.elsevier.com/locate/yseiz Short communication Antipsychotics and seizures: Higher risk with atypicals? a, b c d e Unax Lertxundi *, Rafael Hernandez , Juan Medrano , Saioa Domingo-Echaburu , Monserrat Garcı´a , e Carmelo Aguirre a Pharmacy Service, Red de Salud Mental de Araba, C/alava 43, 01006 Vitoria-Gasteiz, Alava, Spain b Internal Medicine Service, Red de Salud Mental de Araba, Spain c Psychiatry Service, Red de Salud Mental de Araba, Spain d Pharmacy Service, OSI Alto Deba, Spain e Basque Pharmacovigilance Unit, Hospital de Galdakao-Usa´nsolo, Spain A R T I C L E I N F O A B S T R A C T Article history: Purpose: Almost all antipsychotics have been associated with a risk of epileptic seizure provocation. Received 29 May 2012 Among the first-generation antipsychotics (FGA) chlorpromazine appears to be associated with the Received in revised form 17 October 2012 greatest risk of seizures among the second-generation antipsychotics (SGA) clozapine is thought to be Accepted 18 October 2012 most likely to cause convulsions. This information is largely based on studies that are not sufficiently controlled. Besides, information about the seizure risk associated with newer antipsychotics is scarce. Keywords: Method: The Pharmacovigilance Unit of the Basque Country (network of centers of the Spanish Antipsychotics Pharmacovigilance System, SEFV) provided reporting data for adverse reactions (AR) from the whole Seizures SEFV to estimate the reporting odds ratio (ROR) for antipsychotics and seizures (‘‘convulsions’’ as Single Pharmacovigilance MedDra Query). -

Screening of 300 Drugs in Blood Utilizing Second Generation
Forensic Screening of 300 Drugs in Blood Utilizing Exactive Plus High-Resolution Accurate Mass Spectrometer and ExactFinder Software Kristine Van Natta, Marta Kozak, Xiang He Forensic Toxicology use Only Drugs analyzed Compound Compound Compound Atazanavir Efavirenz Pyrilamine Chlorpropamide Haloperidol Tolbutamide 1-(3-Chlorophenyl)piperazine Des(2-hydroxyethyl)opipramol Pentazocine Atenolol EMDP Quinidine Chlorprothixene Hydrocodone Tramadol 10-hydroxycarbazepine Desalkylflurazepam Perimetazine Atropine Ephedrine Quinine Cilazapril Hydromorphone Trazodone 5-(p-Methylphenyl)-5-phenylhydantoin Desipramine Phenacetin Benperidol Escitalopram Quinupramine Cinchonine Hydroquinine Triazolam 6-Acetylcodeine Desmethylcitalopram Phenazone Benzoylecgonine Esmolol Ranitidine Cinnarizine Hydroxychloroquine Trifluoperazine Bepridil Estazolam Reserpine 6-Monoacetylmorphine Desmethylcitalopram Phencyclidine Cisapride HydroxyItraconazole Trifluperidol Betaxolol Ethyl Loflazepate Risperidone 7(2,3dihydroxypropyl)Theophylline Desmethylclozapine Phenylbutazone Clenbuterol Hydroxyzine Triflupromazine Bezafibrate Ethylamphetamine Ritonavir 7-Aminoclonazepam Desmethyldoxepin Pholcodine Clobazam Ibogaine Trihexyphenidyl Biperiden Etifoxine Ropivacaine 7-Aminoflunitrazepam Desmethylmirtazapine Pimozide Clofibrate Imatinib Trimeprazine Bisoprolol Etodolac Rufinamide 9-hydroxy-risperidone Desmethylnefopam Pindolol Clomethiazole Imipramine Trimetazidine Bromazepam Felbamate Secobarbital Clomipramine Indalpine Trimethoprim Acepromazine Desmethyltramadol Pipamperone -

Pharmacology Review(S)
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 200603 PHARMACOLOGY REVIEW(S) Tertiary Pharmacology/Toxicology Review From: Paul C. Brown, Ph.D., ODE Associate Director for Pharmacology and Toxicology, OND IO NDA: 200603 Agency receipt date: 12/30/2009 Drug: Lurasidone hydrochloride Applicant: Sunovion Pharmaceuticals (Originally submitted by Dainippon Sumimoto Pharma America, Inc.) Indication: schizophrenia Reviewing Division: Division of Psychiatry Products Background: The pharm/tox reviewer and team leader concluded that the nonclinical data support approval of lurasidone for the indication listed above. Reproductive and Developmental Toxicity: Reproductive and developmental toxicity studies in rats and rabbits revealed no evidence of teratogenicity or embryofetal toxicity. The high doses in the rat and rabbit embryofetal toxicity studies were 3 and 12 times, respectively, the maximum recommended human dose (80 mg) based on a body surface area comparison. Carcinogenicity: Lurasidone was tested in 2 year rat and mouse carcinogenicity studies. These studies were reviewed by the division and the executive carcinogenicity assessment committee. The committee concluded that the studies were adequate and that there was a drug-related increase in mammary carcinomas in female rats at doses of 12 mg/kg and higher and a drug-related increase in mammary carcinomas and adenoacanthomas and pituitary pars distalis adenomas in female mice. The applicant also provided data from various studies showing that lurasidone significantly increases prolactin in several different species including rats and mice. Conclusions: I agree with the division pharm/tox conclusion that this application can be approved from a pharm/tox perspective. The division recommends that lurasidone be labeled with pregnancy category B. -

Drug Delivery System for Use in the Treatment Or Diagnosis of Neurological Disorders
(19) TZZ __T (11) EP 2 774 991 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 10.09.2014 Bulletin 2014/37 C12N 15/86 (2006.01) A61K 48/00 (2006.01) (21) Application number: 13001491.3 (22) Date of filing: 22.03.2013 (84) Designated Contracting States: • Manninga, Heiko AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 37073 Göttingen (DE) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO •Götzke,Armin PL PT RO RS SE SI SK SM TR 97070 Würzburg (DE) Designated Extension States: • Glassmann, Alexander BA ME 50999 Köln (DE) (30) Priority: 06.03.2013 PCT/EP2013/000656 (74) Representative: von Renesse, Dorothea et al König-Szynka-Tilmann-von Renesse (71) Applicant: Life Science Inkubator Betriebs GmbH Patentanwälte Partnerschaft mbB & Co. KG Postfach 11 09 46 53175 Bonn (DE) 40509 Düsseldorf (DE) (72) Inventors: • Demina, Victoria 53175 Bonn (DE) (54) Drug delivery system for use in the treatment or diagnosis of neurological disorders (57) The invention relates to VLP derived from poly- ment or diagnosis of a neurological disease, in particular oma virus loaded with a drug (cargo) as a drug delivery multiple sclerosis, Parkinsons’s disease or Alzheimer’s system for transporting said drug into the CNS for treat- disease. EP 2 774 991 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 774 991 A1 Description FIELD OF THE INVENTION 5 [0001] The invention relates to the use of virus like particles (VLP) of the type of human polyoma virus for use as drug delivery system for the treatment or diagnosis of neurological disorders. -
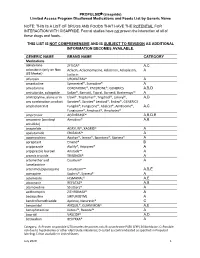
PROPULSID® (Cisapride) Limited Access Program
PROPULSID (cisapride) Limited Access Program Disallowed Medications and Foods List by Generic Name NOTE: THIS IS A LIST OF DRUGS AND FOODS THAT HAVE THE POTENTIAL FOR INTERACTION WITH CISAPRIDE. Formal studies have not proven the interaction of all of these drugs and foods. THIS LIST IS NOT COMPREHENSIVE AND IS SUBJECT TO REVISION AS ADDITIONAL INFORMATION BECOMES AVAILABLE. GENERIC NAME BRAND NAME CATEGORY Medications abiraterone ZYTIGA® A,C aclarubicin (only on Non Aclacin, Aclacinomycine, Aclacinon, Aclaplastin, A US Market) Jaclacin alfuzosin UROXATRAL® A amantadine Symmetrel®, Symadine® A amiodarone CORDARONE®, PACERONE®, GENERICS A,B,D amisulpride, sultopride Solian®, Barnotil, Topral, Barnetil, Barhemsys™ A amitriptyline, alone or in Elavil®, Tryptomer®, Tryptizol®, Laroxyl®, A,D any combination product Saroten®, Sarotex® Lentizol®, Endep®, GENERICS amphotericin B Fungilin®, Fungizone®, Abelcet®, AmBisome®, A,C Fungisome®, Amphocil®, Amphotec® amprenavir AGENERASE® A,B,D,E amsacrine (acridinyl Amsidine® A,B anisidide) anagrelide AGRYLIN®, XAGRID® A apalutamide ERLEADA™ A apomorphine Apokyn®, Ixense®, Spontane®, Uprima® A aprepitant Emend® B aripiprazole Abilify®, Aripiprex® A aripiprazole lauroxil Aristada™ A arsenic trioxide TRISENOX® A artemether and Coartem® A lumefantrine artenimol/piperaquine Eurartesim™ A,B,E asenapine Saphris®, Sycrest® A astemizole HISMANAL® A,E atazanavir REYATAZ® A,B atomoxetine Strattera® A azithromycin ZITHROMAX® A bedaquiline SIRTURO(TM) A bendroflumethiazide Aprinox, Naturetin® C benperidol ANQUIL®, -

Summary of Product Characteristics
NL/H/0662/001/MR NL/H/0663/001/MR NL/H/0664/001/MR SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Tiaprid XXX 100 mg, tablets 2. QUALITATIVE AND QUANTIATIVE COMPOSITION Each tablet contains 100 mg tiapride (as hydrochloride). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet White, round tablets with beveled edge and a cross on both sides. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications For the treatment of neuroleptic-induced tardive dyskinesia, mainly oro-bucco-lingual type. 4.2 Posology and method of administration Adults should take 100-200 mg tiapride three times daily, depending on the severity of the disease and the body weight of the individual . The proposed daily dose for the claimed indication is 300-600 mg tiapride. The effect of treatment may not be apparent until after a period of 4-6 weeks of treatment. Tiaprid XXX tablets should preferably be taken with a little liquid after meals. Tiapride is not intended for treatment in children. Dosage in renal impairment Creatinine clearance: 50-80 ml/min = 75 % of the 10-50 ml/min = 50 % normal less than 10 ml/min = 25 % daily dose 4.3 Contraindications – Hypersensitivity to the active substance or to any of the excipients listed in section 6.1. – Concomitant Prolactin-dependent tumours, e.g. pituitary gland prolactinoma and breast cancer. 1 NL/H/0662/001/MR NL/H/0663/001/MR NL/H/0664/001/MR – Phaeochromocytoma. – Association with levodopa or other dopaminergic medicines (see section 4.5). – Neuroleptic malignant syndrome (see section 4.8). -
Plasma Levels of Long-Acting Injectable Antipsychotics in Outpatient Care: a Retrospective Analysis
Plasma levels of long-acting injectable antipsychotics in outpatient care: a retrospective analysis Martin Hýža Fakultni Nemocnice Ostrava https://orcid.org/0000-0002-2451-2997 Šilhán Petr ( [email protected] ) https://orcid.org/0000-0002-3145-9215 Češková Eva Fakultni Nemocnice Ostrava Skřont Tomáš Fakultni Nemocnice Ostrava Kacířová Ivana Fakultni Nemocnice Ostrava Uřinovská Romana Fakultni Nemocnice Ostrava Grundmann Milan Ostravska Univerzita v Ostrave Research article Keywords: therapeutic drug monitoring, antipsychotics, psychotic disorders, schizophrenia Posted Date: June 9th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-31648/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at Neuropsychiatric Disease and Treatment on April 1st, 2021. See the published version at https://doi.org/10.2147/NDT.S298050. Page 1/10 Abstract Background The antipsychotic ecacy in schizophrenia depends on its availability in the organism. Although therapeutic outcomes remain still far from satisfactory, therapeutic drug monitoring is not a common part of clinical practice during a treatment with long-acting injectable antipsychotics (LAI AP). The real effectiveness of LAI AP is thus uncertain. Methods We made a retrospective evaluation of plasma levels of LAI AP. Collection of blood samples was performed just before the drug application and one week later. Fourty patients with a stabilized clinical condition and steady-state plasma levels were included. Results In the observed cohort of patients, upentixol decanoate (n = 23) was the most often used drug, followed by uphenazine decanoate (n = 7), haloperidol decanoate (n = 5), paliperidon palmitate (n = 3), and risperidone microspheres (n = 2). -

Antipsychotics Therapeutic Class Review (TCR)
Antipsychotics Therapeutic Class Review (TCR) June 9, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. June 2020 Proprietary Information. Restricted Access – Do not disseminate or copy without approval. © 2004 – 2020 Magellan Rx Management. All Rights Reserved. FDA-APPROVED INDICATIONS Indications are for adults unless additional ages are specified. -

Tardive Akathisia
HEALTH & SAFETY: PSYCHIATRIC MEDICATIONS Tardive Akathisia FACT SHEET Tardive Akathisia BQIS Fact Sheets provide a general overview on topics important to supporting an individual’s health and safety and to improving their quality of life. This document provides general information on the topic and is not intended to replace team assessment, decision-making, or medical advice. This is the ninth of ten Fact Sheets regarding psychotropic medications. Intended Outcomes Individuals will understand the symptoms, common causes, and treatment of tardive akathisia. Definitions Akathisia: A movement disorder characterized by motor restlessness/a feeling of inner restlessness with a compelling need to be moving. Tardive akathisia: A severe prolonged form of akathisia which may persist after stopping the medica- tion causing the symptoms. Facts • Akathisia is: – the most common drug induced movement disorder. – a side effect of medication. – most often caused by antipsychotic medications that block dopamine. • Medications with akathisia as a potential side effect include: – Benzisothiazole (ziprasidone) – Benzisoxazole (iloperidone) – Butyrophenones (haloperidol, droperidol) – Calcium channel blockers (flunarizine, cinnarizine) – Dibenzazepine (loxapine, asenapine) – Dibenzodiazepine (clozapine, quetiapine) – Diphenylbutylpiperidine (pimozide) – Indolones (molindone) – Lithium – Phenothiazines (chlorpromazine, triflupromazine, thioridazine, mesoridazine, trifluoperazine, prochlorperazine, perphenazine, fluphenazine, perazine) Bureau of Quality Improvement -

New Information of Dopaminergic Agents Based on Quantum Chemistry Calculations Guillermo Goode‑Romero1*, Ulrika Winnberg2, Laura Domínguez1, Ilich A
www.nature.com/scientificreports OPEN New information of dopaminergic agents based on quantum chemistry calculations Guillermo Goode‑Romero1*, Ulrika Winnberg2, Laura Domínguez1, Ilich A. Ibarra3, Rubicelia Vargas4, Elisabeth Winnberg5 & Ana Martínez6* Dopamine is an important neurotransmitter that plays a key role in a wide range of both locomotive and cognitive functions in humans. Disturbances on the dopaminergic system cause, among others, psychosis, Parkinson’s disease and Huntington’s disease. Antipsychotics are drugs that interact primarily with the dopamine receptors and are thus important for the control of psychosis and related disorders. These drugs function as agonists or antagonists and are classifed as such in the literature. However, there is still much to learn about the underlying mechanism of action of these drugs. The goal of this investigation is to analyze the intrinsic chemical reactivity, more specifcally, the electron donor–acceptor capacity of 217 molecules used as dopaminergic substances, particularly focusing on drugs used to treat psychosis. We analyzed 86 molecules categorized as agonists and 131 molecules classifed as antagonists, applying Density Functional Theory calculations. Results show that most of the agonists are electron donors, as is dopamine, whereas most of the antagonists are electron acceptors. Therefore, a new characterization based on the electron transfer capacity is proposed in this study. This new classifcation can guide the clinical decision‑making process based on the physiopathological knowledge of the dopaminergic diseases. During the second half of the last century, a movement referred to as the third revolution in psychiatry emerged, directly related to the development of new antipsychotic drugs for the treatment of psychosis. -

Longitudinal Evaluation of the Effect of Tricyclic Antidepressants and Neuroleptics on the Course of Huntington’S Disease—Data from a Real World Cohort
brain sciences Article Longitudinal Evaluation of the Effect of Tricyclic Antidepressants and Neuroleptics on the Course of Huntington’s Disease—Data from a Real World Cohort Jannis Achenbach *, Carsten Saft † and Simon Faissner † Huntington Center North Rhine-Westphalia, Department of Neurology, Ruhr-University Bochum, St. Josef-Hospital Bochum, Gudrunstraße 56, 44791 Bochum, Germany; [email protected] (C.S.); [email protected] (S.F.) * Correspondence: [email protected] † These two authors contribute equally to this work. Abstract: Background: Reducing the progress of neurodegeneration is a key goal in Huntington´s disease (HD). A previously performed systematic screening for medications with neuroprotective features identified tricyclic antidepressants and neuroleptics as neuroprotective and mitochondrio- protective agents. Here, we analyzed the characteristics of disease manifestation, progression and potential beneficial effects in HD patients treated with afore-mentioned medications compared to un- and otherwise treated motor-manifest patients in a large real-world cohort over two years. Methods: We analyzed cross-sectional data of the largest cohort worldwide of motor-manifest HD patients using the ENROLL-HD database, including demographic, moleculargenetic, clinical-motoric, cogni- tive and functional data. Longitudinal data of up to two years were obtained to analyze potential Citation: Achenbach, J.; Saft, C.; effects on disease progression between groups with different medications used. Data were analyzed Faissner, S. Longitudinal Evaluation using repeated ANOVA-analyses while controlling for the co-variates age and CAG-repeat length. of the Effect of Tricyclic Results: We identified n = 7397 motor-manifest HD patients using no or different medication (HD- Antidepressants and Neuroleptics on ctrl) and subgroups treated with clomipramine (n = 56), clozapine (n = 66), chlorpromazine (n = 17), the Course of Huntington’s doxepine (n = 34) and desi-, imi- or trimipramine (n = 19). -

3119-3122-Tiapride Is Better Than Risperidone in Treating Senile Dementia
Eur opean Rev iew for Med ical and Pharmacol ogical Sci ences 2016; 20: 3119-3122 Tiapride is more effective and causes fewer adverse effects than risperidone in the treatment of senile dementia Y. YUAN 1, L.H. LI 2, Y.J. HUANG 2, L.F. LEI 2 1Department of Neurology, Xiangya Hospital, Central South University, Changsha, Hunan, China 2Department of Neurology, the 3 rd Xiangya Hospital, Central South University, Changsha, Hunan, China Abstract. – OBJECTIVE: We wanted to com - Risperidone and tiapride are novel antipsychot - pare the effects of tiapride and risperidone in ic drugs and exhibit some advantages in treating treating behavioral and psychological symp - senile dementia 7,8 . Our study aimed at analyzing toms of senile dementia. PATIENTS AND METHODS: 108 patients with the efficacy and safety of these drugs in the treat - senile dementia received respective treatments ment of senile dementia, with the overall goal of (54 patients per treatment, either with 100 providing the reference for drug selection. mg/day risperidone or 2.0 mg tiapride/day) for 2 In the present study, patients with senile demen - months. Outcomes included the positive and tia were treated with either risperidone or tiapride. negative syndrome scale (PANSS) scores, the Our observations demonstrate that tiapride has a curative rate of senile dementia, and prevalence better efficacy and safety profile, than risperidone, of adverse effects (somnolence, headache, loss of weight, extrapyramidal system response, irri - in the treatment of senile dementia. tation and insomnia). RESULTS: PANSS scores before treatment were comparable between treatment groups. On Patients and Methods days 7, 15, 30, and 60 of the treatment, the differ - ences between two treatment groups became Patients evident.