Plasma Levels of Long-Acting Injectable Antipsychotics in Outpatient Care: a Retrospective Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antipsychotics and Seizures: Higher Risk with Atypicals?
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Seizure 22 (2013) 141–143 Contents lists available at SciVerse ScienceDirect Seizure jou rnal homepage: www.elsevier.com/locate/yseiz Short communication Antipsychotics and seizures: Higher risk with atypicals? a, b c d e Unax Lertxundi *, Rafael Hernandez , Juan Medrano , Saioa Domingo-Echaburu , Monserrat Garcı´a , e Carmelo Aguirre a Pharmacy Service, Red de Salud Mental de Araba, C/alava 43, 01006 Vitoria-Gasteiz, Alava, Spain b Internal Medicine Service, Red de Salud Mental de Araba, Spain c Psychiatry Service, Red de Salud Mental de Araba, Spain d Pharmacy Service, OSI Alto Deba, Spain e Basque Pharmacovigilance Unit, Hospital de Galdakao-Usa´nsolo, Spain A R T I C L E I N F O A B S T R A C T Article history: Purpose: Almost all antipsychotics have been associated with a risk of epileptic seizure provocation. Received 29 May 2012 Among the first-generation antipsychotics (FGA) chlorpromazine appears to be associated with the Received in revised form 17 October 2012 greatest risk of seizures among the second-generation antipsychotics (SGA) clozapine is thought to be Accepted 18 October 2012 most likely to cause convulsions. This information is largely based on studies that are not sufficiently controlled. Besides, information about the seizure risk associated with newer antipsychotics is scarce. Keywords: Method: The Pharmacovigilance Unit of the Basque Country (network of centers of the Spanish Antipsychotics Pharmacovigilance System, SEFV) provided reporting data for adverse reactions (AR) from the whole Seizures SEFV to estimate the reporting odds ratio (ROR) for antipsychotics and seizures (‘‘convulsions’’ as Single Pharmacovigilance MedDra Query). -

Comparative Efficacy and Safety of Antipsychotic Drugs for Tic Disorders: a Systematic Review and Bayesian Network Meta-Analysis
Published online: 2018-03-05 Review Thieme Yang Chunsong et al. Comparative Efficacy and Safety … Phar- macopsychiatry 2017; 00: 00–00 Comparative Efficacy and Safety of Antipsychotic Drugs for Tic Disorders: A Systematic Review and Bayesian Network Meta-Analysis Authors Chunsong Yang1,2 * , Zilong Hao3 * , Ling-Li Zhang1,2, Cai-Rong Zhu4, Ping Zhu4, Qin Guo5 Affiliations Supporting Information for this article is available 1 Department of Pharmacy, Evidence-Based Pharmacy online at http:// doi.org/10.1055/s-0043-124872 Center, West China Second Hospital, Sichuan University 2 Key Laboratory of Birth Defects and Related Diseases of ABSTracT Women and Children (Sichuan University), Ministry of Objective The purpose of this study was to evaluate the ef- Education ficacy and safety of antipsychotic drugs for tic disorders (TDs) 3 Department of Neurology, West China Hospital, Sichuan in a network meta-analysis. University, Chengdu, China Methods PubMed, Embase, Cochrane Library, and 4 Chinese 4 West China School of Public Health, Sichuan University databases were searched. Randomized controlled trials (RCTs) 5 Department of Pediatrics, West China Second Hospital, evaluating the efficacy of antipsychotic drugs for TDs were in- Sichuan University cluded. Results Sixty RCTs were included. In terms of tic symptom Key words score, compared with placebo, haloperidol, risperidone, ari- tic disorder, antipsychotic drug, systematic review, network piprazole, quetiapine, olanzapine, and ziprasidone can sig- meta-analysis nificantly improve tic symptom score (standardized mean received 02.09.2017 differences [SMD] ranged from − 12.32 to − 3.20). Quetiapine revised 04.12.2017 was superior to haloperidol, pimozide, risperidone, tiapride, accepted 10.12.2017 aripiprazole, and penfluridol for improving tic symptom score (SMD ranged from − 28.24 to − 7.59). -

The Effects of Antipsychotic Treatment on Metabolic Function: a Systematic Review and Network Meta-Analysis
The effects of antipsychotic treatment on metabolic function: a systematic review and network meta-analysis Toby Pillinger, Robert McCutcheon, Luke Vano, Katherine Beck, Guy Hindley, Atheeshaan Arumuham, Yuya Mizuno, Sridhar Natesan, Orestis Efthimiou, Andrea Cipriani, Oliver Howes ****PROTOCOL**** Review questions 1. What is the magnitude of metabolic dysregulation (defined as alterations in fasting glucose, total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, and triglyceride levels) and alterations in body weight and body mass index associated with short-term (‘acute’) antipsychotic treatment in individuals with schizophrenia? 2. Does baseline physiology (e.g. body weight) and demographics (e.g. age) of patients predict magnitude of antipsychotic-associated metabolic dysregulation? 3. Are alterations in metabolic parameters over time associated with alterations in degree of psychopathology? 1 Searches We plan to search EMBASE, PsycINFO, and MEDLINE from inception using the following terms: 1 (Acepromazine or Acetophenazine or Amisulpride or Aripiprazole or Asenapine or Benperidol or Blonanserin or Bromperidol or Butaperazine or Carpipramine or Chlorproethazine or Chlorpromazine or Chlorprothixene or Clocapramine or Clopenthixol or Clopentixol or Clothiapine or Clotiapine or Clozapine or Cyamemazine or Cyamepromazine or Dixyrazine or Droperidol or Fluanisone or Flupehenazine or Flupenthixol or Flupentixol or Fluphenazine or Fluspirilen or Fluspirilene or Haloperidol or Iloperidone -

Drug Screening: Actual Status, Pitfalls and Suggestions for Improvement Drogenscreening: Gegenwa¨ Rtiger Stand, Fehlermo¨ Glichkeiten Und Verbesserungsvorschla¨Ge
J Lab Med 2004;28(4):317–325 ᮊ 2004 by Walter de Gruyter • Berlin • New York 2004/03304 Drug Monitoring und Toxikologie Redaktion: V. W. Armstrong Drug screening: Actual status, pitfalls and suggestions for improvement Drogenscreening: Gegenwa¨ rtiger Stand, Fehlermo¨ glichkeiten und Verbesserungsvorschla¨ge Wolf R. Ku¨ lpmann* pretierbar. In Wirklichkeit mu¨ ssen viele Aspekte beru¨ ck- sichtigt werden, um einen aussagekra¨ ftigen Befund zu Klinische Chemie, Medizinische Hochschule Hannover, erhalten, z.B. Pra¨ analytik, Spezifita¨ t und Empfindlichkeit Hannover, Germany des Verfahrens oder Qualita¨ tssicherung. Obwohl die Abstract mechanisierten Meßverfahren naturgema¨ ß quantitative Ergebnisse liefern, wird aufgezeigt, daß es sich in der Immunoassays for drug screening are often regarded as Regel empfiehlt, qualitative Befunde zu erstellen. Die procedures which are easily performed and interpreted. Verfahren erlauben aber im Gegensatz zu den auch fu¨r But in fact, many aspects have to be considered to diese Zwecke verwendeten Teststreifen die Entschei- obtain a meaningful result. Among these are sampling, dungsgrenze (cut-off) der jeweiligen Fragestellung anzu- specificity and sensitivity of assays, and quality assess- passen, z.B. bei akuter Intoxikation, chronischem Abusus ment. Although the mechanised procedures yield quan- oder U¨ berwachung des Drogenentzugs. titative results, there are good reasons to generally report Ein schwerwiegender Nachteil von Immunoassays zum qualitative findings. The mechanised procedures allow Nachweis von Amphetamin und a¨ hnlichen Substanzen, the adjustment of the cut-off concentration to the clinical Barbituraten, Benzodiazepinen und Opiaten besteht dar- setting, e.g. in the case of acute intoxication, chronic in, daß eine starke Kreuzreaktivita¨ t des Antiko¨ rpers abuse or drug withdrawal, an important advantage as gegenu¨ ber pharmakologisch wenig wirksamen Verbin- compared to test strips. -

Antipsychotics
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 Class Update with New Drug Evaluations: Antipsychotics Date of Review: May 2016 End Date of Literature Search: February 2016 New Drugs: brexpiprazole Brand Names (Manufacturer): Rexulti® (Otsuka) cariprazine Vraylar™ (Actavis) Dossiers Received: yes PDL Classes: Antipsychotics, First generation Antipsychotics, Second generation Antipsychotics, Parenteral Current Status of PDL Class: See Appendix 1. Purpose for Class Update: Several new antipsychotic drug products have been approved by the U.S. Food and Drug Administration since these drug classes were last reviewed by the Oregon Health Plan (OHP) Pharmacy and Therapeutics Committee. Research Questions: 1. Is there new comparative evidence of meaningful difference in efficacy or effectiveness outcomes for schizophrenia, bipolar mania or major depressive disorders (MDD) between oral antipsychotic agents (first‐ or second‐generation) or between parenteral antipsychotic agents (first‐ or second‐generation)? 2. Is there new comparative evidence of meaningful difference in harms between oral antipsychotic agents (first‐ or second‐generation) or between parenteral antipsychotic agents (first‐ or second‐generation)? 3. Is there new comparative evidence of meaningful difference in effectiveness or harms in certain subpopulations based on demographic characteristics? Conclusions: There is insufficient evidence of clinically meaningful differences between antipsychotic agents in efficacy or effectiveness or harms between antipsychotic agents for schizophrenia, bipolar mania or MDD. There is insufficient evidence to determine if brexpiprazole and cariprazine offer superior efficacy or safety to other antipsychotic agents for schizophrenia. There is insufficient evidence to determine if brexpiprazole offers superior efficacy or safety to other antipsychotic agents for MDD. -

Screening of 300 Drugs in Blood Utilizing Second Generation
Forensic Screening of 300 Drugs in Blood Utilizing Exactive Plus High-Resolution Accurate Mass Spectrometer and ExactFinder Software Kristine Van Natta, Marta Kozak, Xiang He Forensic Toxicology use Only Drugs analyzed Compound Compound Compound Atazanavir Efavirenz Pyrilamine Chlorpropamide Haloperidol Tolbutamide 1-(3-Chlorophenyl)piperazine Des(2-hydroxyethyl)opipramol Pentazocine Atenolol EMDP Quinidine Chlorprothixene Hydrocodone Tramadol 10-hydroxycarbazepine Desalkylflurazepam Perimetazine Atropine Ephedrine Quinine Cilazapril Hydromorphone Trazodone 5-(p-Methylphenyl)-5-phenylhydantoin Desipramine Phenacetin Benperidol Escitalopram Quinupramine Cinchonine Hydroquinine Triazolam 6-Acetylcodeine Desmethylcitalopram Phenazone Benzoylecgonine Esmolol Ranitidine Cinnarizine Hydroxychloroquine Trifluoperazine Bepridil Estazolam Reserpine 6-Monoacetylmorphine Desmethylcitalopram Phencyclidine Cisapride HydroxyItraconazole Trifluperidol Betaxolol Ethyl Loflazepate Risperidone 7(2,3dihydroxypropyl)Theophylline Desmethylclozapine Phenylbutazone Clenbuterol Hydroxyzine Triflupromazine Bezafibrate Ethylamphetamine Ritonavir 7-Aminoclonazepam Desmethyldoxepin Pholcodine Clobazam Ibogaine Trihexyphenidyl Biperiden Etifoxine Ropivacaine 7-Aminoflunitrazepam Desmethylmirtazapine Pimozide Clofibrate Imatinib Trimeprazine Bisoprolol Etodolac Rufinamide 9-hydroxy-risperidone Desmethylnefopam Pindolol Clomethiazole Imipramine Trimetazidine Bromazepam Felbamate Secobarbital Clomipramine Indalpine Trimethoprim Acepromazine Desmethyltramadol Pipamperone -

Emea/666243/2009
European Medicines Agency London, 29 October 2009 EMEA/666243/2009 ISSUE NUMBER: 0910 MONTHLY REPORT PHARMACOVIGILANCE WORKING PARTY (PHVWP) OCTOBER 2009 PLENARY MEETING The CHMP Pharmacovigilance Working Party (PhVWP) held its October 2009 plenary meeting on 19-21 October 2009. PhVWP DISCUSSIONS ON SAFETY CONCERNS Below is a summary of the discussions regarding non-centrally authorised medicinal products in accordance with the PhVWP publication policy (see under http://www.emea.europa.eu/htms/human/phv/reports.htm). Positions agreed by the PhVWP for non- centrally authorised products are recommendations to Member States. For safety updates concerning centrally authorised products and products subject to ongoing CHMP procedures, readers are referred to the CHMP Monthly Report (see under http://www.emea.europa.eu/pressoffice/presshome.htm). The PhVWP provides advice on these products to the Committee of Medicinal Products for Human Use (CHMP) upon its request. Antipsychotics - risk of venous thromboembolism (VTE) Identify risk factors for VTE for preventive action before and during treatment with antipsychotics The PhVWP completed their review on the risk of VTE of antipsychotics1. The review was triggered by and based on data from the UK spontaneous adverse drug reactions reporting system and the published literature. The PhVWP carefully considered the data, including the limitations of both information sources, such as the lack of randomised controlled trial data, the heterogeneity of published studies and the potential confounding factors such as sedation and weight gain, commonly present in antipsychotic users. The PhVWP concluded that an association between VTE and antipsychotics cannot be excluded. Distinguishing different risk levels between the various active substances was not possible. -

Pharmacokinetic Considerations in Antipsychotic Augmentation Strategies: How to Combine Risperidone with Low-Potency Antipsychotics
Progress in Neuro-Psychopharmacology & Biological Psychiatry 76 (2017) 101–106 Contents lists available at ScienceDirect Progress in Neuro-Psychopharmacology & Biological Psychiatry journal homepage: www.elsevier.com/locate/pnp Pharmacokinetic considerations in antipsychotic augmentation strategies: How to combine risperidone with low-potency antipsychotics Michael Paulzen a,b,1, Georgios Schoretsanitis a,b,f,⁎,1, Benedikt Stegmann d,ChristophHiemkeg,h, Gerhard Gründer a,b,KoenR.J.Schruerse, Sebastian Walther f, Sarah E. Lammertz a,b,EkkehardHaenc,d a Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Aachen, Germany b JARA – Translational Brain Medicine, Aachen, Germany c Clinical Pharmacology, Department of Psychiatry and Psychotherapy, University of Regensburg, Regensburg, Germany d Department of Pharmacology and Toxicology, University of Regensburg, Regensburg, Germany e Faculty of Health, Medicine and Life Sciences, School for Mental Health and Neuroscience, Maastricht University, Maastricht, Netherlands f University Hospital of Psychiatry, Bern, Switzerland g Department of Psychiatry and Psychotherapy, University Medical Center of Mainz, Germany h Institute of Clinical Chemistry and Laboratory Medicine, University Medical Center of Mainz, Germany article info abstract Article history: Objectives: To investigate in vivo the effect of low-potency antipsychotics on metabolism of risperidone (RIS). Received 22 December 2016 Methods: A therapeutic drug monitoring database containing plasma concentrations of RIS and its metabolite 9-OH- Accepted 12 March 2017 RIS of 1584 patients was analyzed. Five groups were compared; a risperidone group (n = 842) and four co- med- Available online 14 March 2017 ication groups; a group co-medicated with chlorprothixene (n = 67), a group with levomepromazine (n = 32), a group with melperone (n = 46), a group with pipamperone (n = 63) and a group with prothipendyl (n = 24). -

1.25.3 Boden2012b
Clinical evidence – study characteristics tables 1.25.3 BODEN2012B Study ID BODEN2012B Bibliographic reference Boden R, Lundgren M, Brandt L, Reutfors J, Andersen M, Kieler H. Risks of adverse pregnancy and birth outcomes in women treated or not treated with Antenatal and postnatal mental health (update) 319 Clinical evidence – study characteristics tables mood stabilisers for bipolar disorder: Population based cohort study. BMJ (Online). 2012b;345 Methods Prospective/Retrospective: Prospective Data collection: Registries Country: SE Participants Trimester of exposure: Not reported Duration of exposure: Not reported Total N: 358203 N Exposed: 507 N Unexposed: 357696 Mean age (years): 14.6% <25; 63.7% 25-34; 21.7% = >35. Diagnosis: Exposed groups: 90.3% any psychiatric diagnosis; 20.9% schizophrenia; 17.6% other nonaffective psychosis; 11.2% bipolar disorder. Non-exposed group: 8.7% any psychiatric diagnosis; 0.03% schizophrenia; 0.1% other nonaffective psychosis; 0.2% bipolar disorder. Inclusion criteria: All women giving birth in Sweden from July 1, 2005, through December 31, 2009, grouped by filled prescriptions for (1) olanzapine and/or clozapine, the most obesogenic and diabetogenic antipsychotics, (2) other antipsychotics, or (3) no antipsychotics. Exposure was defined as filling a prescription for an antipsychotic (Anatomical Therapeutic Chemical code N05A) from last menstrual period to parturition. Exclusion criteria: Excluded prochlorperazine, levomepromazine, and melperone prescriptions because these drugs are mainly used as antiemetics or anxiolytics with low and intermittently administrated doses. Lithium, which also belongs to the Anatomical Therapeutic Chemical category N05A,was excluded because of its different pharmacological action and placental passage compared with the other compounds in the N05A group and because it is mainly used to treat bipolar disorder. -

Pharmacology Review(S)
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 200603 PHARMACOLOGY REVIEW(S) Tertiary Pharmacology/Toxicology Review From: Paul C. Brown, Ph.D., ODE Associate Director for Pharmacology and Toxicology, OND IO NDA: 200603 Agency receipt date: 12/30/2009 Drug: Lurasidone hydrochloride Applicant: Sunovion Pharmaceuticals (Originally submitted by Dainippon Sumimoto Pharma America, Inc.) Indication: schizophrenia Reviewing Division: Division of Psychiatry Products Background: The pharm/tox reviewer and team leader concluded that the nonclinical data support approval of lurasidone for the indication listed above. Reproductive and Developmental Toxicity: Reproductive and developmental toxicity studies in rats and rabbits revealed no evidence of teratogenicity or embryofetal toxicity. The high doses in the rat and rabbit embryofetal toxicity studies were 3 and 12 times, respectively, the maximum recommended human dose (80 mg) based on a body surface area comparison. Carcinogenicity: Lurasidone was tested in 2 year rat and mouse carcinogenicity studies. These studies were reviewed by the division and the executive carcinogenicity assessment committee. The committee concluded that the studies were adequate and that there was a drug-related increase in mammary carcinomas in female rats at doses of 12 mg/kg and higher and a drug-related increase in mammary carcinomas and adenoacanthomas and pituitary pars distalis adenomas in female mice. The applicant also provided data from various studies showing that lurasidone significantly increases prolactin in several different species including rats and mice. Conclusions: I agree with the division pharm/tox conclusion that this application can be approved from a pharm/tox perspective. The division recommends that lurasidone be labeled with pregnancy category B. -
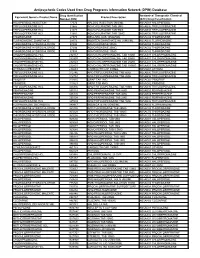
Antipsychotic Codes Used from Drug Programs Information Network (DPIN) Database
Antipsychotic Codes Used from Drug Programs Information Network (DPIN) Database Drug Identification Anatomical Therapeutic Chemical Equivalent Generic Product Name Product Description Number (DIN) (ATC) Drug Classification HALOPERIDOL INJECTION 17574 HALDOL INJECTION 5MG/ML N05AD01 HALOPERIDOL TRIFLUOPERAZINE HCL 21865 NOVO-FLURAZINE TAB 2MG N05AB06 TRIFLUOPERAZINE TRIFLUOPERAZINE HCL 21873 NOVO-FLURAZINE TAB 5MG N05AB06 TRIFLUOPERAZINE TRIFLUOPERAZINE HCL 21881 NOVO-FLURAZINE TAB 10MG N05AB06 TRIFLUOPERAZINE THIORIDAZINE 27375 MELLARIL SUS 10MG/5ML N05AC02 THIORIDAZINE FLUPHENAZINE ENANTHATE 29173 MODITEN ENANTHATE INJ 25MG/ML N05AB02 FLUPHENAZINE THIORIDAZINE HYDROCHLORIDE 37486 NOVO-RIDAZINE 50MG N05AC02 THIORIDAZINE THIORIDAZINE HYDROCHLORIDE 37494 NOVO-RIDAZINE 25MG N05AC02 THIORIDAZINE THIORIDAZINE HYDROCHLORIDE 37508 NOVO-RIDAZINE 10MG N05AC02 THIORIDAZINE CHLORPROMAZINE HCL 232157 NOVO-CHLORPROMAZINE TAB 10MG N05AA01 CHLORPROMAZINE CHLORPROMAZINE HCL 232807 NOVO-CHLORPROMAZINE TAB 50MG N05AA01 CHLORPROMAZINE CHLORPROMAZINE HCL 232823 NOVO-CHLORPROMAZINE TAB 25MG N05AA01 CHLORPROMAZINE CHLORPROMAZINE HCL 232831 NOVO-CHLORPROMAZINE TAB 100MG N05AA01 CHLORPROMAZINE LITHIUM CARBONATE 236683 CARBOLITH CAP 300MG N05AN01 LITHIUM TRIFLUOPERAZINE HCL 312746 APO TRIFLUOPERAZINE TAB 5MG N05AB06 TRIFLUOPERAZINE TRIFLUOPERAZINE HCL 312754 APO TRIFLUOPERAZINE TAB 2MG N05AB06 TRIFLUOPERAZINE PIMOZIDE 313815 ORAP TAB 2MG N05AG02 PIMOZIDE PIMOZIDE 313823 ORAP TAB 4MG N05AG02 PIMOZIDE TRIFLUOPERAZINE HCL 326836 APO TRIFLUOPERAZINE TAB 10MG N05AB06 -

Drug Delivery System for Use in the Treatment Or Diagnosis of Neurological Disorders
(19) TZZ __T (11) EP 2 774 991 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 10.09.2014 Bulletin 2014/37 C12N 15/86 (2006.01) A61K 48/00 (2006.01) (21) Application number: 13001491.3 (22) Date of filing: 22.03.2013 (84) Designated Contracting States: • Manninga, Heiko AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 37073 Göttingen (DE) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO •Götzke,Armin PL PT RO RS SE SI SK SM TR 97070 Würzburg (DE) Designated Extension States: • Glassmann, Alexander BA ME 50999 Köln (DE) (30) Priority: 06.03.2013 PCT/EP2013/000656 (74) Representative: von Renesse, Dorothea et al König-Szynka-Tilmann-von Renesse (71) Applicant: Life Science Inkubator Betriebs GmbH Patentanwälte Partnerschaft mbB & Co. KG Postfach 11 09 46 53175 Bonn (DE) 40509 Düsseldorf (DE) (72) Inventors: • Demina, Victoria 53175 Bonn (DE) (54) Drug delivery system for use in the treatment or diagnosis of neurological disorders (57) The invention relates to VLP derived from poly- ment or diagnosis of a neurological disease, in particular oma virus loaded with a drug (cargo) as a drug delivery multiple sclerosis, Parkinsons’s disease or Alzheimer’s system for transporting said drug into the CNS for treat- disease. EP 2 774 991 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 774 991 A1 Description FIELD OF THE INVENTION 5 [0001] The invention relates to the use of virus like particles (VLP) of the type of human polyoma virus for use as drug delivery system for the treatment or diagnosis of neurological disorders.