1.25.3 Boden2012b
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effects of Antipsychotic Treatment on Metabolic Function: a Systematic Review and Network Meta-Analysis
The effects of antipsychotic treatment on metabolic function: a systematic review and network meta-analysis Toby Pillinger, Robert McCutcheon, Luke Vano, Katherine Beck, Guy Hindley, Atheeshaan Arumuham, Yuya Mizuno, Sridhar Natesan, Orestis Efthimiou, Andrea Cipriani, Oliver Howes ****PROTOCOL**** Review questions 1. What is the magnitude of metabolic dysregulation (defined as alterations in fasting glucose, total cholesterol, low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, and triglyceride levels) and alterations in body weight and body mass index associated with short-term (‘acute’) antipsychotic treatment in individuals with schizophrenia? 2. Does baseline physiology (e.g. body weight) and demographics (e.g. age) of patients predict magnitude of antipsychotic-associated metabolic dysregulation? 3. Are alterations in metabolic parameters over time associated with alterations in degree of psychopathology? 1 Searches We plan to search EMBASE, PsycINFO, and MEDLINE from inception using the following terms: 1 (Acepromazine or Acetophenazine or Amisulpride or Aripiprazole or Asenapine or Benperidol or Blonanserin or Bromperidol or Butaperazine or Carpipramine or Chlorproethazine or Chlorpromazine or Chlorprothixene or Clocapramine or Clopenthixol or Clopentixol or Clothiapine or Clotiapine or Clozapine or Cyamemazine or Cyamepromazine or Dixyrazine or Droperidol or Fluanisone or Flupehenazine or Flupenthixol or Flupentixol or Fluphenazine or Fluspirilen or Fluspirilene or Haloperidol or Iloperidone -

Drug Screening: Actual Status, Pitfalls and Suggestions for Improvement Drogenscreening: Gegenwa¨ Rtiger Stand, Fehlermo¨ Glichkeiten Und Verbesserungsvorschla¨Ge
J Lab Med 2004;28(4):317–325 ᮊ 2004 by Walter de Gruyter • Berlin • New York 2004/03304 Drug Monitoring und Toxikologie Redaktion: V. W. Armstrong Drug screening: Actual status, pitfalls and suggestions for improvement Drogenscreening: Gegenwa¨ rtiger Stand, Fehlermo¨ glichkeiten und Verbesserungsvorschla¨ge Wolf R. Ku¨ lpmann* pretierbar. In Wirklichkeit mu¨ ssen viele Aspekte beru¨ ck- sichtigt werden, um einen aussagekra¨ ftigen Befund zu Klinische Chemie, Medizinische Hochschule Hannover, erhalten, z.B. Pra¨ analytik, Spezifita¨ t und Empfindlichkeit Hannover, Germany des Verfahrens oder Qualita¨ tssicherung. Obwohl die Abstract mechanisierten Meßverfahren naturgema¨ ß quantitative Ergebnisse liefern, wird aufgezeigt, daß es sich in der Immunoassays for drug screening are often regarded as Regel empfiehlt, qualitative Befunde zu erstellen. Die procedures which are easily performed and interpreted. Verfahren erlauben aber im Gegensatz zu den auch fu¨r But in fact, many aspects have to be considered to diese Zwecke verwendeten Teststreifen die Entschei- obtain a meaningful result. Among these are sampling, dungsgrenze (cut-off) der jeweiligen Fragestellung anzu- specificity and sensitivity of assays, and quality assess- passen, z.B. bei akuter Intoxikation, chronischem Abusus ment. Although the mechanised procedures yield quan- oder U¨ berwachung des Drogenentzugs. titative results, there are good reasons to generally report Ein schwerwiegender Nachteil von Immunoassays zum qualitative findings. The mechanised procedures allow Nachweis von Amphetamin und a¨ hnlichen Substanzen, the adjustment of the cut-off concentration to the clinical Barbituraten, Benzodiazepinen und Opiaten besteht dar- setting, e.g. in the case of acute intoxication, chronic in, daß eine starke Kreuzreaktivita¨ t des Antiko¨ rpers abuse or drug withdrawal, an important advantage as gegenu¨ ber pharmakologisch wenig wirksamen Verbin- compared to test strips. -

Antipsychotics
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 Class Update with New Drug Evaluations: Antipsychotics Date of Review: May 2016 End Date of Literature Search: February 2016 New Drugs: brexpiprazole Brand Names (Manufacturer): Rexulti® (Otsuka) cariprazine Vraylar™ (Actavis) Dossiers Received: yes PDL Classes: Antipsychotics, First generation Antipsychotics, Second generation Antipsychotics, Parenteral Current Status of PDL Class: See Appendix 1. Purpose for Class Update: Several new antipsychotic drug products have been approved by the U.S. Food and Drug Administration since these drug classes were last reviewed by the Oregon Health Plan (OHP) Pharmacy and Therapeutics Committee. Research Questions: 1. Is there new comparative evidence of meaningful difference in efficacy or effectiveness outcomes for schizophrenia, bipolar mania or major depressive disorders (MDD) between oral antipsychotic agents (first‐ or second‐generation) or between parenteral antipsychotic agents (first‐ or second‐generation)? 2. Is there new comparative evidence of meaningful difference in harms between oral antipsychotic agents (first‐ or second‐generation) or between parenteral antipsychotic agents (first‐ or second‐generation)? 3. Is there new comparative evidence of meaningful difference in effectiveness or harms in certain subpopulations based on demographic characteristics? Conclusions: There is insufficient evidence of clinically meaningful differences between antipsychotic agents in efficacy or effectiveness or harms between antipsychotic agents for schizophrenia, bipolar mania or MDD. There is insufficient evidence to determine if brexpiprazole and cariprazine offer superior efficacy or safety to other antipsychotic agents for schizophrenia. There is insufficient evidence to determine if brexpiprazole offers superior efficacy or safety to other antipsychotic agents for MDD. -

Screening of 300 Drugs in Blood Utilizing Second Generation
Forensic Screening of 300 Drugs in Blood Utilizing Exactive Plus High-Resolution Accurate Mass Spectrometer and ExactFinder Software Kristine Van Natta, Marta Kozak, Xiang He Forensic Toxicology use Only Drugs analyzed Compound Compound Compound Atazanavir Efavirenz Pyrilamine Chlorpropamide Haloperidol Tolbutamide 1-(3-Chlorophenyl)piperazine Des(2-hydroxyethyl)opipramol Pentazocine Atenolol EMDP Quinidine Chlorprothixene Hydrocodone Tramadol 10-hydroxycarbazepine Desalkylflurazepam Perimetazine Atropine Ephedrine Quinine Cilazapril Hydromorphone Trazodone 5-(p-Methylphenyl)-5-phenylhydantoin Desipramine Phenacetin Benperidol Escitalopram Quinupramine Cinchonine Hydroquinine Triazolam 6-Acetylcodeine Desmethylcitalopram Phenazone Benzoylecgonine Esmolol Ranitidine Cinnarizine Hydroxychloroquine Trifluoperazine Bepridil Estazolam Reserpine 6-Monoacetylmorphine Desmethylcitalopram Phencyclidine Cisapride HydroxyItraconazole Trifluperidol Betaxolol Ethyl Loflazepate Risperidone 7(2,3dihydroxypropyl)Theophylline Desmethylclozapine Phenylbutazone Clenbuterol Hydroxyzine Triflupromazine Bezafibrate Ethylamphetamine Ritonavir 7-Aminoclonazepam Desmethyldoxepin Pholcodine Clobazam Ibogaine Trihexyphenidyl Biperiden Etifoxine Ropivacaine 7-Aminoflunitrazepam Desmethylmirtazapine Pimozide Clofibrate Imatinib Trimeprazine Bisoprolol Etodolac Rufinamide 9-hydroxy-risperidone Desmethylnefopam Pindolol Clomethiazole Imipramine Trimetazidine Bromazepam Felbamate Secobarbital Clomipramine Indalpine Trimethoprim Acepromazine Desmethyltramadol Pipamperone -

Emea/666243/2009
European Medicines Agency London, 29 October 2009 EMEA/666243/2009 ISSUE NUMBER: 0910 MONTHLY REPORT PHARMACOVIGILANCE WORKING PARTY (PHVWP) OCTOBER 2009 PLENARY MEETING The CHMP Pharmacovigilance Working Party (PhVWP) held its October 2009 plenary meeting on 19-21 October 2009. PhVWP DISCUSSIONS ON SAFETY CONCERNS Below is a summary of the discussions regarding non-centrally authorised medicinal products in accordance with the PhVWP publication policy (see under http://www.emea.europa.eu/htms/human/phv/reports.htm). Positions agreed by the PhVWP for non- centrally authorised products are recommendations to Member States. For safety updates concerning centrally authorised products and products subject to ongoing CHMP procedures, readers are referred to the CHMP Monthly Report (see under http://www.emea.europa.eu/pressoffice/presshome.htm). The PhVWP provides advice on these products to the Committee of Medicinal Products for Human Use (CHMP) upon its request. Antipsychotics - risk of venous thromboembolism (VTE) Identify risk factors for VTE for preventive action before and during treatment with antipsychotics The PhVWP completed their review on the risk of VTE of antipsychotics1. The review was triggered by and based on data from the UK spontaneous adverse drug reactions reporting system and the published literature. The PhVWP carefully considered the data, including the limitations of both information sources, such as the lack of randomised controlled trial data, the heterogeneity of published studies and the potential confounding factors such as sedation and weight gain, commonly present in antipsychotic users. The PhVWP concluded that an association between VTE and antipsychotics cannot be excluded. Distinguishing different risk levels between the various active substances was not possible. -

Pharmacokinetic Considerations in Antipsychotic Augmentation Strategies: How to Combine Risperidone with Low-Potency Antipsychotics
Progress in Neuro-Psychopharmacology & Biological Psychiatry 76 (2017) 101–106 Contents lists available at ScienceDirect Progress in Neuro-Psychopharmacology & Biological Psychiatry journal homepage: www.elsevier.com/locate/pnp Pharmacokinetic considerations in antipsychotic augmentation strategies: How to combine risperidone with low-potency antipsychotics Michael Paulzen a,b,1, Georgios Schoretsanitis a,b,f,⁎,1, Benedikt Stegmann d,ChristophHiemkeg,h, Gerhard Gründer a,b,KoenR.J.Schruerse, Sebastian Walther f, Sarah E. Lammertz a,b,EkkehardHaenc,d a Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Aachen, Germany b JARA – Translational Brain Medicine, Aachen, Germany c Clinical Pharmacology, Department of Psychiatry and Psychotherapy, University of Regensburg, Regensburg, Germany d Department of Pharmacology and Toxicology, University of Regensburg, Regensburg, Germany e Faculty of Health, Medicine and Life Sciences, School for Mental Health and Neuroscience, Maastricht University, Maastricht, Netherlands f University Hospital of Psychiatry, Bern, Switzerland g Department of Psychiatry and Psychotherapy, University Medical Center of Mainz, Germany h Institute of Clinical Chemistry and Laboratory Medicine, University Medical Center of Mainz, Germany article info abstract Article history: Objectives: To investigate in vivo the effect of low-potency antipsychotics on metabolism of risperidone (RIS). Received 22 December 2016 Methods: A therapeutic drug monitoring database containing plasma concentrations of RIS and its metabolite 9-OH- Accepted 12 March 2017 RIS of 1584 patients was analyzed. Five groups were compared; a risperidone group (n = 842) and four co- med- Available online 14 March 2017 ication groups; a group co-medicated with chlorprothixene (n = 67), a group with levomepromazine (n = 32), a group with melperone (n = 46), a group with pipamperone (n = 63) and a group with prothipendyl (n = 24). -
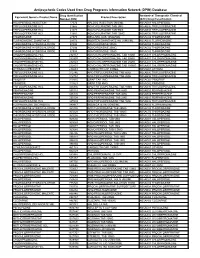
Antipsychotic Codes Used from Drug Programs Information Network (DPIN) Database
Antipsychotic Codes Used from Drug Programs Information Network (DPIN) Database Drug Identification Anatomical Therapeutic Chemical Equivalent Generic Product Name Product Description Number (DIN) (ATC) Drug Classification HALOPERIDOL INJECTION 17574 HALDOL INJECTION 5MG/ML N05AD01 HALOPERIDOL TRIFLUOPERAZINE HCL 21865 NOVO-FLURAZINE TAB 2MG N05AB06 TRIFLUOPERAZINE TRIFLUOPERAZINE HCL 21873 NOVO-FLURAZINE TAB 5MG N05AB06 TRIFLUOPERAZINE TRIFLUOPERAZINE HCL 21881 NOVO-FLURAZINE TAB 10MG N05AB06 TRIFLUOPERAZINE THIORIDAZINE 27375 MELLARIL SUS 10MG/5ML N05AC02 THIORIDAZINE FLUPHENAZINE ENANTHATE 29173 MODITEN ENANTHATE INJ 25MG/ML N05AB02 FLUPHENAZINE THIORIDAZINE HYDROCHLORIDE 37486 NOVO-RIDAZINE 50MG N05AC02 THIORIDAZINE THIORIDAZINE HYDROCHLORIDE 37494 NOVO-RIDAZINE 25MG N05AC02 THIORIDAZINE THIORIDAZINE HYDROCHLORIDE 37508 NOVO-RIDAZINE 10MG N05AC02 THIORIDAZINE CHLORPROMAZINE HCL 232157 NOVO-CHLORPROMAZINE TAB 10MG N05AA01 CHLORPROMAZINE CHLORPROMAZINE HCL 232807 NOVO-CHLORPROMAZINE TAB 50MG N05AA01 CHLORPROMAZINE CHLORPROMAZINE HCL 232823 NOVO-CHLORPROMAZINE TAB 25MG N05AA01 CHLORPROMAZINE CHLORPROMAZINE HCL 232831 NOVO-CHLORPROMAZINE TAB 100MG N05AA01 CHLORPROMAZINE LITHIUM CARBONATE 236683 CARBOLITH CAP 300MG N05AN01 LITHIUM TRIFLUOPERAZINE HCL 312746 APO TRIFLUOPERAZINE TAB 5MG N05AB06 TRIFLUOPERAZINE TRIFLUOPERAZINE HCL 312754 APO TRIFLUOPERAZINE TAB 2MG N05AB06 TRIFLUOPERAZINE PIMOZIDE 313815 ORAP TAB 2MG N05AG02 PIMOZIDE PIMOZIDE 313823 ORAP TAB 4MG N05AG02 PIMOZIDE TRIFLUOPERAZINE HCL 326836 APO TRIFLUOPERAZINE TAB 10MG N05AB06 -

The Effect of the Process Variables on the HPLC Separation of Tricyclic Neuroleptics on a Calixarene-Bonded Stationary Phase
ORIGINAL ARTICLES Institute of Pharmacy, Pharmaceutical/Medicinal Chemistry, Ernst-Moritz-Arndt-University Greifswald, Greifswald, Germany The effect of the process variables on the HPLC separation of tricyclic neuroleptics on a calixarene-bonded stationary phase H. Hashem, Th. Jira Received March 15, 2004, accepted May 25, 2004 Priv.-Doz. Dr. Thomas Jira, Ernst-Moritz-Arndt-University Greifswald, Institute of Pharmacy, Pharmaceu- tical/Medicinal Chemistry, F.-L.-Jahnstr. 17, D-17487 Greifswald, Germany [email protected] Pharmazie 60: 186–192 (2005) The chromatographic behavior of a new HPLC-stationary phase with supramolecular selectors on the basis of calixarenes is described for the separation of nine tricyclic neuroleptics. The effects of differ- ent chromatographic conditions (buffer system, pH-value, type and content of organic modifier, injec- tion volume) on the separation of the analytes were studied. Additionally, the effect of structural differ- ences of the neuroleptic analytes was studied. The chemical structure and pKa of the neuroleptics highly influenced their separation on the calix[8]arene phase. The separation of all analytes on the investigated calixarene-bonded stationary phase was possible with a mobile phase of acetonitrile with 30 mM ammonium acetate buffer (pH 3.5) 30 : 70(v/v) using 1 ml/min flow rate. 1. Introduction Neuroleptics which are widely used for the treatment of CH3 psychological problems are basic compounds, mainly thiox- CH3 anthene and phenothiazine derivatives. Many papers de- N N CH3 scribe the separation of this pharmacological group of ana- N N CH3 lytes on the usual RP-stationary phases (Goldstein and Van S CH3 S O Vunakis 1981; Kountourellis and Markopoulou 1991; Trac- CH3 qui et al. -

Antipsychotics-2020.Pdf
© Mind 2020 Antipsychotics Explains what antipsychotics are used for, how the medication works, possible side effects and information about withdrawal. If you require this information in Word document format for compatibility with screen readers, please email: [email protected] Contents What are antipsychotics? ...................................................................................................................... 2 Could antipsychotics help me? .............................................................................................................. 5 How to take antipsychotics safely ......................................................................................................... 9 What dosage of antipsychotics should I be on? .................................................................................. 15 Antipsychotics during pregnancy and breastfeeding ........................................................................... 17 What side effects can antipsychotics cause? ....................................................................................... 20 What is a depot injection? ................................................................................................................... 30 How can I compare different antipsychotics? ..................................................................................... 31 Can I come off antipsychotics? ............................................................................................................ 41 Alternatives to antipsychotics -

Ecological Assessment of Clinicians' Antipsychotic Prescription Habits In
JOURNAL OF MEDICAL INTERNET RESEARCH Berrouiguet et al Original Paper Ecological Assessment of Clinicians' Antipsychotic Prescription Habits in Psychiatric Inpatients: A Novel Web- and Mobile Phone±Based Prototype for a Dynamic Clinical Decision Support System Sofian Berrouiguet1,2,3, MD; Maria Luisa Barrigón4, MD, PhD; Sara A Brandt5, MSc; George C Nitzburg5, PhD; Santiago Ovejero4, MD; Raquel Alvarez-Garcia4, MD; Juan Carballo4, MD, PhD; Michel Walter1, MD, PhD; Romain Billot2, PhD; Philippe Lenca6, PhD; David Delgado-Gomez7, PhD; Juliette Ropars8, MD; Ivan de la Calle Gonzalez9, MEng; Philippe Courtet10, MD, PhD; Enrique Baca-García4, MD, PhD 1Department of Psychiatry, Brest Medical University Hospital at Brest, Brest, France 2UMR CNRS 6285 Lab-STICC, Institut Mines-Telecom, Brest, France 3ERCR SPURBO, Université de Bretagne occidentale, Brest, France 4Department of Psychiatry, Fundacion Jimenez Diaz Hospital, Madrid, Spain 5Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, United States 6IMT Atlantique, UMR CNRS 6285 Lab-STICC, Technopôle Brest Iroise, Brest, France 7Departamento de Estadistica, Universidad Carlos III de Madrid, Madrid, Spain 8LaTIM - INSERM UMR 1101, Brest Medical University Hospital, Brest, France 9Cabaro Soluciones SA, Valladolid, Spain 10INSERM U1061, Université de Montpellier, Montpellier, France Corresponding Author: Sofian Berrouiguet, MD Department of Psychiatry Brest Medical University Hospital at Brest Urgences Psychiatrques CHRU Brest Brest, 29200 France Phone: 33 668204178 Fax: 33 298015218 Email: [email protected] Abstract Background: Electronic prescribing devices with clinical decision support systems (CDSSs) hold the potential to significantly improve pharmacological treatment management. Objective: The aim of our study was to develop a novel Web- and mobile phone±based application to provide a dynamic CDSS by monitoring and analyzing practitioners' antipsychotic prescription habits and simultaneously linking these data to inpatients' symptom changes. -
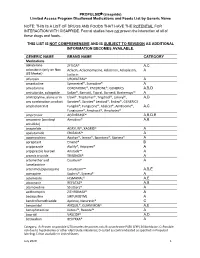
PROPULSID® (Cisapride) Limited Access Program
PROPULSID (cisapride) Limited Access Program Disallowed Medications and Foods List by Generic Name NOTE: THIS IS A LIST OF DRUGS AND FOODS THAT HAVE THE POTENTIAL FOR INTERACTION WITH CISAPRIDE. Formal studies have not proven the interaction of all of these drugs and foods. THIS LIST IS NOT COMPREHENSIVE AND IS SUBJECT TO REVISION AS ADDITIONAL INFORMATION BECOMES AVAILABLE. GENERIC NAME BRAND NAME CATEGORY Medications abiraterone ZYTIGA® A,C aclarubicin (only on Non Aclacin, Aclacinomycine, Aclacinon, Aclaplastin, A US Market) Jaclacin alfuzosin UROXATRAL® A amantadine Symmetrel®, Symadine® A amiodarone CORDARONE®, PACERONE®, GENERICS A,B,D amisulpride, sultopride Solian®, Barnotil, Topral, Barnetil, Barhemsys™ A amitriptyline, alone or in Elavil®, Tryptomer®, Tryptizol®, Laroxyl®, A,D any combination product Saroten®, Sarotex® Lentizol®, Endep®, GENERICS amphotericin B Fungilin®, Fungizone®, Abelcet®, AmBisome®, A,C Fungisome®, Amphocil®, Amphotec® amprenavir AGENERASE® A,B,D,E amsacrine (acridinyl Amsidine® A,B anisidide) anagrelide AGRYLIN®, XAGRID® A apalutamide ERLEADA™ A apomorphine Apokyn®, Ixense®, Spontane®, Uprima® A aprepitant Emend® B aripiprazole Abilify®, Aripiprex® A aripiprazole lauroxil Aristada™ A arsenic trioxide TRISENOX® A artemether and Coartem® A lumefantrine artenimol/piperaquine Eurartesim™ A,B,E asenapine Saphris®, Sycrest® A astemizole HISMANAL® A,E atazanavir REYATAZ® A,B atomoxetine Strattera® A azithromycin ZITHROMAX® A bedaquiline SIRTURO(TM) A bendroflumethiazide Aprinox, Naturetin® C benperidol ANQUIL®, -

Second Generation Anti-Psychotic Drugs Effect on Negative Symptoms of Schizophrenia Versus First Generation
Drug Information Center Second generation anti-psychotic drugs effect on negative symptoms of schizophrenia versus first generation Schizophrenia is a severe brain disorder in which people interpret reality abnormally. Schizophrenia may result in some combination of hallucinations, delusions, and extremely disordered thinking and behavior. Schizophrenia involves a range of problems with thinking (cognitive), behavior or emotions (1) . Signs and symptoms of schizophrenia include positive and negative symptoms. Positive symptoms: delusion, hallucination, disorganized thinking (speech) and abnormal motor behavior. Negative symptoms effect on ability to function normally (lack emotion , lack eye contact , flat effect , decease talking , loss of interest and decrease the self-care ) (1). Schizophrenia treated with antipsychotic and other agents like antidepressants and mood stabilizers (lithium, carbamazepine, valproic acids). Antipsychotics work by altering the effect of certain chemicals in the brain, called dopamine, serotonin, noradrenalin and acetylcholine that effect on patient behavior, mood and emotions. (2) There are two types of antipsychotic: First generation (typical antipsychotic): chlorpromazine, flupentixol, haloperidol, levomepromazine, pericyazine, perphenazine, pimozide, sulpiride, trifluoperazine, and zuclopenthixol. Second generation (atypical antipsychotic): amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, risperidone and sertindole.(2) The differences between first and second generation antipsychotic are