Dossier – Mineral Oil Hydrocarbons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HISTORY of WESTERN OIL SHALE HISTORY of WESTERN OIL SHALE
/ _... i';C4 - SHELF , Historyof Western Oil Shale Paul L. Russell . " The Center for Professional Advancement Paul Russell received his degree from the University of Arizona. After working for Industry for five years, he began his involvement with oil shale in 1948 when he joined the U.S. Bureau of Mines and was assigned to Rifle, Colorado, to work at Anvil Points. During the middle fifties, he was assigned to the Atomic Energy Com mission to study the extraction of ura nium from the Chattanooga Shales in Tennessee. He became Research Director of the U.S. Bureau ofMines in 1967 and served in this capacity until he retired in 1979. During these years his involvement with oil shale intensified. Currently, he is an engineering consultant. ISBN: 0-86563-000-3 ,._-------_._.. V.D.ALLRED 6016 SOUTH BANNOCK LI7TLETON. COLO. 80120 ....~ ...........~..... This compelling history spans 65 years of western oil shale development from its begin ning to the present day. These were the years in which most of the present-day retorting pro cesses were invented and devel oped,leading to present studies of in-situ retorting, and to the resumption of leasing of fed eral oil shale lands. The many excellent illustra tions and contemporary photo graphs in themselves provide a pictorial record of an era when the United States was "wild over oil"-an era when Gov ernment estimates of billions of barrels of oil in western oil shales were used to advan tage for questionable-if not fraudulent-stock promotions designed to raise capital for development, or to fatten the promoters' pockets. -

Mineral Oil (Medium Viscosity)
MINERAL OIL (MEDIUM VISCOSITY) Prepared at the 76th JECFA, published in FAO JECFA Monographs 13 (2012), superseding specifications for Mineral oil (Medium and low viscosity), class I prepared at the 59th JECFA (2002), published in FNP 52 Add 10 (2002) and republished in FAO JECFA Monographs 1 (2005). An ADI of 0-10 mg/kg bw was established at the 59th JECFA for mineral oil (medium and low), class I. At the 76th JECFA the temporary ADI and the specifications for mineral oils (Medium and low viscosity), class II and class III were withdrawn. SYNONYMS Liquid paraffin, liquid petrolatum, food grade mineral oil, white mineral oil, INS No. 905e DEFINITION A mixture of highly refined paraffinic and naphthenic liquid hydrocarbons with boiling point above 200°; obtained from mineral crude oils through various refining steps (eg. distillation, extraction and crystallisation) and subsequent purification by acid and/or catalytic hydrotreatment; may contain antioxidants approved for food use. C.A.S. number 8012-95-1 DESCRIPTION Colourless, transparent, oily liquid, free from fluorescence in daylight; odourless FUNCTIONAL USES Release agent, glazing agent CHARACTERISTICS IDENTIFICATION Solubility (Vol. 4) Insoluble in water, sparingly soluble in ethanol, soluble in ether Burning Burns with bright flame and with paraffin-like characteristic smell PURITY Viscosity, 100° 8.5-11 mm2/s See description under TESTS Carbon number at 5% Not less than 25 distillation point The boiling point at the 5% distillation point is higher than: 391°. See description under TESTS Average molecular 480-500 weight See description under TESTS Acidity or alkalinity To 10 ml of the sample add 20 ml of boiling water and shake vigorously for 1 min. -

Salicylic Acid
Treatment Guide to Common Skin Conditions Prepared by Loren Regier, BSP, BA, Sharon Downey -www.RxFiles.ca Revised: Jan 2004 Dermatitis, Atopic Dry Skin Psoriasis Step 1 - General Treatment Measures Step 1 - General Treatment Measures Step 1 • Avoid contact with irritants or trigger factors • Use cool air humidifiers • Non-pharmacologic measures (general health issues) • Avoid wool or nylon clothing. • Lower house temperature (minimize perspiration) • Moisturizers (will not clear skin, but will ↓ itching) • Wash clothing in soap vs detergent; double rinse/vinegar • Limit use of soap to axillae, feet, and groin • Avoid frequent or prolonged bathing; twice weekly • Topical Steroids Step 2 recommended but daily bathing permitted with • Coal Tar • Colloidal oatmeal bath products adequate skin hydration therapy (apply moisturizer • Anthralin • Lanolin-free water miscible bath oil immediately afterwards) • Vitamin D3 • Intensive skin hydration therapy • Limit use of soap to axillae, feet, and groin • Topical Retinoid Therapy • “Soapless” cleansers for sensitive skin • Apply lubricating emollients such as petrolatum to • Sunshine Step 3 damp skin (e.g. after bathing) • Oral antihistamines (1st generation)for sedation & relief of • Salicylic acid itching give at bedtime +/- a daytime regimen as required Step 2 • Bath additives (tar solns, oils, oatmeal, Epsom salts) • Topical hydrocortisone (0.5%) for inflammation • Colloidal oatmeal bath products Step 2 apply od-tid; ointments more effective than creams • Water miscible bath oil • Phototherapy (UVB) may use cream during day & ointment at night • Humectants: urea, lactic acid, phospholipid • Photochemotherapy (Psoralen + UVA) Step 4 Step 3 • Combination Therapies (from Step 1 & 2 treatments) • Prescription topical corticosteroids: use lowest potency • Oral antihistamines for sedation & relief of itching steroid that is effective and wean to twice weekly. -

Safety Data Sheet According to 29CFR1910/1200 and GHS Rev
Safety Data Sheet according to 29CFR1910/1200 and GHS Rev. 3 Effective date : 02.09.2015 Page 1 of 6 Mineral Oil,Light SECTION 1 : Identification of the substance/mixture and of the supplier Product name : Mineral Oil,Light Manufacturer/Supplier Trade name: Manufacturer/Supplier Article number: S25439 Recommended uses of the product and uses restrictions on use: Manufacturer Details: AquaPhoenix Scientific 9 Barnhart Drive, Hanover, PA 17331 Supplier Details: Fisher Science Education 15 Jet View Drive, Rochester, NY 14624 Emergency telephone number: Fisher Science Education Emergency Telephone No.: 800-535-5053 SECTION 2 : Hazards identification Classification of the substance or mixture: Not classified for physical or health hazards according to GHS. Hazard statements: Precautionary statements: If medical advice is needed, have product container or label at hand Keep out of reach of children Read label before use Other Non-GHS Classification: WHMIS NFPA/HMIS NFPA SCALE (0-4) HMIS RATINGS (0-4) SECTION 3 : Composition/information on ingredients Ingredients: CAS 8042-47-5 Mineral Oil 100 % Created by Global Safety Management, Inc. -Tel: 1-813-435-5161 - www.gsmsds.com Safety Data Sheet according to 29CFR1910/1200 and GHS Rev. 3 Effective date : 02.09.2015 Page 2 of 6 Mineral Oil,Light Percentages are by weight SECTION 4 : First aid measures Description of first aid measures After inhalation: Loosen clothing as necessary and position individual in a comfortable position.Move exposed to fresh air. Give artificial respiration if necessary. If breathing is difficult give oxygen.Get medical assistance if cough or other symptoms appear. After skin contact: Rinse/flush exposed skin gently using soap and water for 15-20 minutes.Seek medical advice if discomfort or irritation persists. -

Anisol - a Convenient Immersion Medium for Microscopy
CONVENIENT IMMERSION MEDIUM TOR MICROSCOPY 151 Anisol - a Convenient Immersion Medium for Microscopy by Dr L. J. BRUCE-CHWATr, Chief, Planning Section, Division of Malaria Eradication, World Health Organization, Geneva During the surveillance phase of malaria eradication the examination of blood for malaria parasites is of paramount importance. In the early stage of surveillance the amount of work connected with the collection and examination of very numerous blood slides is so great that it often constitutes a major bottle-neck of the programme. The re-examination of all or a proportion of positive blood films by a central laboratory is an accepted feature of a well-organized programme of malaria eradication. Any improvements in the microscopical technique of blood examination are therefore of considerable value. One of the most common immersion media used for the examination of blood slides with high-power lenses is cedar oil, obtained from the juniper tree (Juniperus virginiana), commonly known by the wrong name of " red cedar ". The thickened oil has a refractive index of 1.515, which is very close to that of the glass of which high-powered objectives are made. The refractive index of the glass used for slides and coverslips is about 1.520. There are slight differences in the refractive indices of cedar oils produced by different manufacturers, and some manufacturers have produced proprietary oils of standard composition which are less subject to drying than cedar oil itself. Several microscopists have recommended other immersion media, such as mixtures of resin and linseed oil, castor oil, glycerin, etc. Rowntree a advised the use of heavy mineral oil, otherwise known as liquid paraffin or petrolatum, which has a lower refractive index (1.470) and should, for best observation, contain 12 %-18 % a-bromonaphthalene (Jensenb). -

Celebrating the Rich History of Waxes Bladel, the Netherlands What’S Inside: Watertown, Connecticut, Usa
CELEBRATING THE RICH HISTORY OF WAXES BLADEL, THE NETHERLANDS WHAT’S INSIDE: WATERTOWN, CONNECTICUT, USA 2-3 – HERITAGE 4-5 – INNOVATION 6-7 – WORLD RESOURCES 8-9 – NATURAL/ORGANIC 10-11 – SILICONYL WAXES 12-13 – CUSTOM BLENDS 14-15 – EMULSIFYING WAXES 16-17 – KESTER WAXES 18-19 – MILKS 20-41 – WAX SPECIFICATIONS 42 – WAX PROPERTIES KOSTER WAX FACT: Koster Keunen was founded in the Netherlands and is world renowned for supplying quality waxes. 1852 OUR HISTORY OF TRADITION AND INNOVATION Founded in 1852 as a family business, Koster Keunen has evolved into the world’s leading processor, refiner and marketer of natural waxes. From the early days of sun bleaching beeswax for the candle industry, we now specialize in processing and formulating quality waxes for cosmetics, pharmaceutical, food, coatings, and various other technical industries worldwide. For over 150 years we have sought perfection, constantly introducing new and innovative processes and waxes, while investing in experienced, knowledgeable people and the best equipment to help meet this goal. As a family business we believe very strongly in the need for developing 3 superior quality products, and supporting our customers with excellent service, throughout the formulation and marketing processes. From our two facilities, in the USA and Holland, we offer a huge range of natural waxes, synthetic waxes and wax derivatives, enabling our customers to produce thousands of products that look, feel and work superbly KOSTERKEUNEN.COM / 1 860.945.3333 KOSTER WAX FACT: Koster Keunen was the first natural wax company to manufacture waxes using a Sandvik Pastillator, starting in 1988. 1852 UNIQUELY KOSTER KEUNEN Our greatest strength is the experience and scientific expertise we have fostered for the development of new and innovative products. -

Safety Data Sheet
SAFETY DATA SHEET 1. Identification Product identifier Mineral Oil Other means of identification Catalog number 1443952 Chemical name n/f Recommended use Specified quality tests and assay use only. Recommended restrictions Not for use as a drug. Not for administration to humans or animals. Manufacturer/Importer/Supplier/Distributor information Company name U. S. Pharmacopeia Address 12601 Twinbrook Parkway Rockville MD 20852-1790 US Telephone RS Technical Services 301-816-8129 Website www.usp.org E-mail [email protected] Emergency phone number CHEMTREC within US & 1-800-424-9300 Canada CHEMTREC outside US & +1 703-527-3887 Canada 2. Hazard(s) identification Physical hazards Not classified. Health hazards Serious eye damage/eye irritation Category 2A Aspiration hazard Category 1 OSHA hazard(s) Not classified. Label elements Signal word Danger Hazard statement Causes serious eye irritation. May be fatal if swallowed and enters airways. Precautionary statement Prevention Wash thoroughly after handling. Wear eye/face protection. Response If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. If swallowed: Immediately call a poison center/doctor. Do NOT induce vomiting. Storage Store locked up. Disposal Dispose of contents/container in accordance with local/regional/national/international regulations. Hazard(s) not otherwise Not classified. classified (HNOC) 3. Composition/information on ingredients Substance Hazardous components Chemical name Common name and synonyms CAS number % Mineral Oil 8012-95-1 100 4. First-aid measures Inhalation Move to fresh air. Call a physician if symptoms develop or persist. -
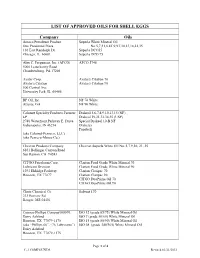
LIST of APPROVED OILS for SHELL EGGS Company Oils
LIST OF APPROVED OILS FOR SHELL EGGS Company Oils Amoco Petroleum Product Superla White Mineral Oil One Prudential Plaza No.5,7,31,6,8T,9,9T,10,13,18,21,35 130 East Randolph Dr Superla DCO55 Chicago, IL 60601 Superla DCO 75 Alex C. Ferguuson, Inc. (AFCO) AFCO 5746 5000 Letterkenny Road Chambersburg, PA 17201 Avatar Corp. Avatar's Citation 70 Avatar's Citation Avatar's Citation 90 500 Central Ave University Park, IL 60466 BP Oil, Inc. NF 70 White Atlanta, GA NF 90 White Calumet Specialty Products Partners Drakeol 5,6,7,8,9,10,13,15 (NF) LP Drakeol 19,21,32,34,35 (USP) 2780 Waterfront Parkway E. Drive Special Drakeol 10-B NF Indianapolis, IN 46214 Draketex Peneteck (aka Calumet-Penreco, LLC) (aka Penreco-Morco Co.) Chevron Products Company Chevron Superla White Oil No. 5,7,9,10, 21, 35 6101 Bollinger Canyon Road San Ramon, CA 94583 CITGO Petroleum Corp. Clarion Food Grade White Mineral 70 Lubricant Division Clarion Food Grade White Mineral 90 1293 Eldridge Parkway Clarion Claripac 70 Houston, TX 77077 Clarion Claripac 90 CITGO DuoPrime Oil 70 CITGO DuoPrime Oil 90 Chute Chemical Co Solvent 170 233 Bomarc Rd Bangor, ME 04401 Conoco-Phillips Company600 N. ISO 12 (grade 65/75) White Mineral Oil Dairy Ashford ISO 7 (grade 50/60) White Mineral Oil Houston, TX 77079-1175 ISO 15 (grade 80/90) White Mineral Oil (aka “Phillips 66”, “76 Lubricants”) ISO 68 (grade 340/365) White Mineral Oil Dairy Ashford Houston, TX 77079-1175 Page 1 of 4 C-3 COMPOUNDS Revised 03/21/2012 Durvet Durvet Mineral Oil 100 S.E. -

Three Great Finishes (That Aren't Polyurethane)
Three great finishes (that aren't polyurethane) Many woodworkers choose polyurethane as a go-to finish simply for its familiarity. Easy to apply, it looks good on a variety of woods and provides plenty of protection. But poly may not always be the best choice. Consider one of these three other clear finishes for your next project. You'll be glad you did. Danish oil: Smooth and easy When it comes to bringing out the natural beauty of a highly figured piece of wood, such as quilted maple or quartersawn oak, nothing beats a hand-rubbed Danish-oil finish. Typically a Danish oil consists of a mixture of tung oil and varnish. It penetrates into the wood, unlike a film finish, which sits on the surface. That penetration gives a depth to the wood's grain that's hard to achieve with a film finish. Applying Danish oil is simple -- you dip a cloth in the finish, then use it to flood the wood's surface, photo below Let it soak in for 15 minutes, and work more into areas that absorb the oil. These spots will appear dull. Then, wipe off any excess because "puddles" dry tacky. This "bleedback" occurs particularly in open-grained woods, such as red oak. Make it satin smooth Danish oil dries slowly, so wait overnight before recoating. And it goes on thin, so apply a minimum of three coats. You don't have to worry about brush marks, but you'll get an even smoother finish by lightly "wet" sanding between the second and third coats. -

2,000 Trees a Day: Work and Life in the American Naval Stores Industry, 1877 to 1940 by Catherine Kim Gyllerstrom a Dissertatio
2,000 Trees a Day: Work and Life in the American Naval Stores Industry, 1877 to 1940 by Catherine Kim Gyllerstrom A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama December 13, 2014 Keywords: Naval Stores, Turpentine, African American, Labor, Convict Lease, Debt Peonage Copyright 2014 by Catherine Kim Gyllerstrom Approved by Jennifer Brooks, Chair, Professor of History Ruth Crocker, Professor of History Angela Lakwete, Professor of History Tiffany Sippial, Professor of History Abstract This project explores the lives of nineteenth and early twentieth century naval stores workers in Alabama, Georgia, and Florida. After the Civil War, turpentine operators faced a high demand for their product, limited capital to embark on new operations, and an uncertain labor supply. Therefore, these men resorted to deceitful labor recruitment tactics to entice free workers to their camps. In addition, operators also supplemented their work force with convict labor. The preliminary focus of this dissertation is the experience—nature of work, work culture, and daily life—of turpentine employees. Previous historians, with the exception of Robert Outland, have dismissed turpentine harvesting as a makeshift operation on the periphery of civilization. In turn, this assessment has led to the misconception that turpentine workers were wild and violent frontiersmen, who rarely formed social bonds, idolized outlaws, and ascribed to a rough and tumble way of life. This work seeks to restore the reputation of naval stores laborers and contends that these men—both African American and white, both free and captive—shared a similar work culture to other industrial workers and established and supported families within the camps. -

Wax-Oil Lubricants to Reduce the Shear Between Skin and PPE
Wax-Oil Lubricants to Reduce the Shear Between Skin and PPE Kian Kun Yap ( [email protected] ) Imperial College London Manoj Murali Imperial College London Zhengchu Tan University of Oxford Xue Zhou Southwest Jiaotong University Luli Li Imperial College London Marc Masen Imperial College London Research Article Keywords: Wax-oil lubricants, PPE, COVID-19, skin Posted Date: April 13th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-403040/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/18 Abstract Prolonged use of tight-tting PPE, e.g., by COVID-19 healthcare workers leads to skin injuries. An important contributor is the shear exerted on the skin due to static friction at the skin-PPE interface. This study aims to develop an optimised wax-oil lubricant that reduces the friction, or shear, in the skin-PPE contact for up to four hours. Lubricants with different wax-oil combinations were prepared using beeswax, paran wax, olive oil, and mineral oil. In-vivo friction measurements involving seven participants were conducted by sliding a polydimethylsiloxane ball against the volar forearms to simulate the skin-PPE interface. The maximum static coecient of friction was measured immediately and four hours after lubricant application. It was found that the coecient of friction of wax-oil lubricants is mainly governed by the ratio of wax to oil and the thermal stability and morphology of the wax. To maintain long-term lubricity, it is crucial to consider the absorption of oil into the PPE material. -

Mineral Oil – Origin, Production and Composition
Mineral oil – Origin, production and composition Anna Hedelin, Nynas AB for CONCAWE 10 September 2013 Reproduction permitted with due acknowledgement What is Mineral Oil? Wikipedia IARC Petroleum industry Origin non-vegetable (mineral) prepared from naturally occurring Obtained from crude oil crude petroleum oil Production a distillate of petroleum Crude oil is distilled first at atmospheric The chemical composition is set by pressure and then under high vacuum to manufacturing processes to satisfy a yield vacuum distillates and residual range of performance, physical and fractions that can be further refined to toxicological properties mineral oils Apperance colorless, odorless Hydrocarbon 15-30 >15 15-50 no range Boiling point 300-600 range (°C) Molecular mixtures of alkanes complex and variable mixtures of Complex substances of hydrocarbon structures straight and branched-chain paraffinic, components; consists of alkanes naphthenic (isoparaffincs), saturated cyclic (cycloparaffinic), and aromatic alkanes (naphthenics), alkylated hydrocarbons aromatics Synonyms imprecise, having been base oils, mineral Often considered as lubricant base used to label many specific base oils or lubricant base oils oils or white oils oils over the past few centuries. Other names, similarly imprecise, include white oil, liquid paraffin, Reproduction permitted with due acknowledgement and liquid petroleum. Baby oil refers to a perfumed MOCRINIS work-shop,mineral Bolognaoil. Sept 10-11, 2013 Mineral oil - definition No clear definition Petroleum industry: