Phenolic-Extract from Argan Oil (Argania Spinosa L.) Inhibits Human
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Factors Affecting Invasive Species Abundance: the Barbary Ground Squirrel on Fuerteventura Island, Spain Marta López-Darias1,* and Jorge M
Zoological Studies 47(3): 268-281 (2008) Factors Affecting Invasive Species Abundance: the Barbary Ground Squirrel on Fuerteventura Island, Spain Marta López-Darias1,* and Jorge M. Lobo2 1Departamento de Biología Aplicada, Estación Biológica de Doñana (CSIC), Pabellón del Perú, Avenida María Luisa s/n, Sevilla, E-41013, Spain 2Departamento de Biodiversidad y Biología Evolutiva, Museo Nacional de Ciencias Naturales (CSIC), Calle José Gutiérrez Abascal 2, Madrid, E-28006, Spain. E-mail:[email protected] (Accepted November 14, 2007) Marta López-Darias and Jorge M. Lobo (2008) Factors affecting invasive species abundance: the Barbary ground squirrel on Fuerteventura Island, Spain. Zoological Studies 47(3): 268-281. We assessed the determinants of habitat selection by the Barbary ground squirrel (Atlantoxerus getulus) at Fuerteventura (Canary Is., Spain). We implemented general linear model (GLM) procedures to analyze the relationships between squirrel abundances and 4 kinds of variables related to the biological requirements of the species (environment, food resources, biotic interactions, and refuge/shelter). We performed a variance partitioning analysis between the most explicative categories to explore correlation patterns. The time of year and weather conditions of the census clearly influenced the number of individuals observed. Shelter variables were the best correlates of both the abundance of squirrels and the number of their scat. Although food resources were less important, the presence of certain plant species was correlated with squirrel abundance, while general environmental variables and interactions with other mammals did not affect its distribution. These results improve our understanding of the ecology and the establishment of this highly successful introduced species, providing basic knowledge for use with future management strategies in the Canarian Archipelago. -

Ethnoeconomical, Ethnomedical, and Phytochemical Study of Argania Spinosa (L.) Skeels: a Review
ELSEVIER - Journal of Ethnopharmacology Review Article Ethnoeconomical, Ethnomedical, and Phytochemical Study of Argania spinosa (L.) Skeels: A Review. Zoubida Charrouf Dominique Guillaume ABSTRACT. Populations of Morocco South-western part traditionally use the fruits of Argania spinosa (L.) Skeels to prepare an edible oil whose obtention also furnishes, as side product, a cake used to feed the cattle and complements the forage furnished by the leaves of this same plant. Unfortunately, the wood of Argania spinosa is also used for fuel and deforestation is subsequently accelerated since populations are generally eager to replace argan groves by cultures of higher and immediate benefit. However, argan tree, that is particularly well adapted to grow in arid lands, has been proposed by several agencies to slow down the desert progress in northern Africa. In order to incite the South-western Morocco dwellers to reintroduce argan trees, a program aimed to increase the industrial value of Argania spinosa, and beginning by its phytochemical study, is currently carried out in Morocco. The results of these recent studies together with previous knowledge are summarised in this review. KEYWORDS. Morocco, Argania spinosa, argan tree, argan oil, ethnopharmacology, phytochemistry, saponins INTRODUCTION Argan tree (Argania spinosa (L.) Skeels), of the family sapotaceae, is endemic in South- western Morocco where it grows over about 320,000 square miles. For centuries, this slow growing and spiny tree, that may be either shrubby or up to seven or ten meters has played an essential ecological function in this part of Morocco. Indeed, it effectively protects the soil against heavy rain or wind-induced erosion and, furthermore, by shading all kind of cultures, maintains soil fertility. -

Comparative Study of Argan and Olive Fruits and Oils
435 Comparative study of argan and olive fruits and oils Dalila Demnati1, Sebastián Sánchez2, Rafael Pacheco2, Mohamed Zahar1 & Leopoldo Martínez2 1- Institut Agronomique et Vétérinaire Hassan. BP 6202, 10101, Rabat, Morocco. Email: [email protected] 2- Universidad de Jaén, Campus Las Lagunillas 23071 Jaén, Spain. Email: [email protected] Abstract This study was conducted to compare argan and olive fruits and virgin oils. Dry argan fruits, traditional and semiautomatic extracted argan oils, from roasted and unroasted seeds, from Essaouira’s area, were studied. Morphological characteristics of argan fruit were determined and compared with the ‘Picual’ olive’s ones. The results showed certain similarities between the two fruits. The quality parameters analyzed were acidity and peroxide value, K270, K232 and ΔK, total phenols and oil stability, comparing them with those of ‘Picual’ virgin olive oil. Quality parameters corresponded to the Moroccan Standard for edible virgin argan oil. Traditional argan oil showed the lowest stability whereas semiautomatic edible oil presented the highest one. However, virgin olive oil showed higher phenol content and better oxidative stability than the virgin argan oils. Keywords: Argan, Olive, Fruit, Quality Parameters, Phenols, Oil Stability. Etude comparative des caractéristiques des fruits et des huiles d’argan et d’olive Résumé Cette étude a pour but de comparer le fruit et l’huile d’argan et d’olive vierges. Les fruits secs d’arganier, les huiles d’argan obtenues par un procédé traditionnel ou semi-automatique, à partir d’amandons torréfiés ou non, de la région d’Essaouira, ont été étudiés. Les caractéristiques morphologiques du fruit de l’arganier ont été déterminées et comparées à ceux de l’olive, variété ‘Picual’. -

ISTA List of Stabilised Plant Names 7Th Edition
ISTA List of Stabilised Plant Names 7th Edition ISTA Nomenclature Committee Chair Dr. M. Schori Published by All rights reserved. No part of this publication may be The International Seed Testing Association (ISTA) reproduced, stored in any retrieval system or transmitted in Richtiarkade 18, CH- 8304 Wallisellen, Switzerland any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior ©2021 International Seed Testing Association (ISTA) permission in writing from ISTA. ISBN 978-3-906549-77-4 Valid from: 16.06.2021 ISTA List of Stabilised Plant Names 1st Edition 1966 ISTA Nomenclature Committee Chair: Prof P. A. Linehan 2nd Edition 1983 ISTA Nomenclature Committee Chair: Dr. H. Pirson 3rd Edition 1988 ISTA Nomenclature Committee Chair: Dr. W. A. Brandenburg 4th Edition 2001 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 5th Edition 2007 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 6th Edition 2013 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 7th Edition 2019 ISTA Nomenclature Committee Chair: Dr. M. Schori 7th Edition 2 ISTA List of Stabilised Plant Names Table of Contents A .............................................................................................................................................................. 7 B ............................................................................................................................................................ 21 C ........................................................................................................................................................... -

Argania Spinosa)
plants Article Isolation and Functional Analysis of a PISTILLATA-like MADS-Box Gene from Argan Tree (Argania spinosa) Marwa Louati 1 , Blanca Salazar-Sarasua 2 , Edelín Roque 2, José Pío Beltrán 2, Amel Salhi Hannachi 1 and Concepción Gómez-Mena 2,* 1 Faculty of Sciences of Tunis, Campus Farhat Hached El Manar, University of Tunis El Manar, Tunis 2092, Tunisia; [email protected] (M.L.); [email protected] (A.S.H.) 2 Instituto de Biología Molecular y Celular de Plantas, Consejo Superior de Investigaciones Científicas, Universitat Politècnica de València, 46022 Valencia, Spain; [email protected] (B.S.-S.); [email protected] (E.R.); [email protected] (J.P.B.) * Correspondence: [email protected] Abstract: Argan trees (Argania spinosa) belong to a species native to southwestern Morocco, playing an important role in the environment and local economy. Argan oil extracted from kernels has a unique composition and properties. Argan trees were introduced in Tunisia, where hundreds of trees can be found nowadays. In this study, we examined reproductive development in Argan trees from four sites in Tunisia and carried out the functional characterization of a floral homeotic gene in this non-model species. Despite the importance of reproductive development, nothing is known about the genetic network controlling flower development in Argania spinosa. Results obtained in several plant species established that floral organ development is mostly controlled by MADS-box genes and, in particular, APETALA3 (AP3) and PISTILLATA (PI) homologs are required for proper petal and stamen identity. Here, we describe the isolation and functional characterization Citation: Louati, M.; Salazar-Sarasua, B.; Roque, E.; Beltrán, J.P.; Salhi of a MADS-box gene from Argania spinosa. -

Facts and Misconceptions on the Palaearctic Existence of the Striped
Mammalia 2017; aop Boris Kryštufek, Cătălin Stanciu, Danijel Ivajnšič*, Sidi Imad Cherkaoui and Franc Janžekovič Facts and misconceptions on the Palaearctic existence of the striped ground squirrel https://doi.org/10.1515/mammalia-2017-0060 echo the exclusive ecological requirements of species Received May 26, 2017; accepted July 27, 2017 and their evolutionary history in response to past eco- logical and geological processes (Lomolino et al. 2006). Abstract: The striped ground squirrel has a wide distri- Species’ ranges can be studied at various temporal and bution in the Ethiopian region but is restricted to a small spatial scales provided they are already documented. isolated area in Palaearctic Africa. This fragment was first Before a distributional map can be produced, data on recorded in the late 1940s in the Souss Valley (Morocco), spatial occurrence must be collected in the field. Despite however, not a single new observation has been published its obvious simplicity, field work constitutes a crucial step in the following decades. In September 2016 we surveyed and affects the consistency of analyses which may depend the Souss Valley and found squirrels at 43 sites within upon sophisticated tools and concepts. Incomplete or the triangle between Agadir–Taroudant–Tiznit. Occupied misleading distributional data will unavoidably compro- sites were not distributed at random but occurred between mise subsequent analyses and assessments. an altitude of 45–254 m and on a substrate with coarse tex- In this study we have addressed the only Palaearctic ture containing >65% sand. The vast majority of the sites occurrence of the striped ground squirrel Euxerus with squirrels (69%) were classified as suburban, culti- erythropus (Geoffroy Saint-Hilaire 1803) (formerly Xerus vated or both. -

CIRCULAR Issue No
FDACS-P-01915 CIRCULAR Issue No. 40 | October 2018 Florida Department of Agriculture and Consumer Services Division of Plant Industry The Buckthorns (Genus Sideroxylon): An Underappreciated Group of Florida Native Plants Paul T. Corogin; Bureau of Entomology, Nematology and Plant Pathology [email protected] or 1-888-397-1517 INTRODUCTION Tucked away amongst the rich diversity of Florida plant life surrounding us, one plant group can easily escape our notice: the genus Sideroxylon, belonging to the pantropical family Sapotaceae (sapodilla family). This circular will introduce the Sideroxylon species native to North America, featuring in detail species adapted to the temperate zone that may be of interest to the southern United States (U.S.). Some are endangered in Florida, and some are Florida endemics. Certain species have landscaping potential, but have long been ignored, but a few species are occasionally available from native plant nurseries (Betrock’s Plant Search 2018; FNPS 2018). Species of Sideroxylon attract pollinators when blooming, and birds and wildlife when fruiting; thus, they can be desirable additions to any Florida landscape. Sapotaceae are recognized by the presence of milky sap, brownish T-shaped hairs, fasciculate inflorescences (flowers in a bundle) and seeds with a large scar at one end (Pennington 1990, 1991). This woody family makes a large contribution to tropical plant biodiversity, being a major floristic component of tropical lowland wet forests in the Americas, Asia, Africa and the Pacific Islands (Gentry 1988). Sapotaceous plants are also economically important to humans. “Sapote” comes from the Nahuatl word meaning sweet fruit; most species bear such a fruit (e.g., the sapodilla and mamey sapote) (Smith et al. -

Download Download
BORN IN THE MEDITERRANEAN: Alicia Vicente,3 Ma Angeles´ Alonso,3 and COMPREHENSIVE TAXONOMIC Manuel B. Crespo3* REVISION OF BISCUTELLA SER. BISCUTELLA (BRASSICACEAE) BASED ON MORPHOLOGICAL AND PHYLOGENETIC DATA1,2 ABSTRACT Biscutella L. ser. Biscutella (5 Biscutella ser. Lyratae Malin.) comprises mostly annual or short-lived perennial plants occurring in the Mediterranean basin and the Middle East, which exhibit some diagnostic floral features. Taxa in the series have considerable morphological plasticity, which is not well correlated with clear geographic or ecologic patterns. Traditional taxonomic accounts have focused on a number of vegetative and floral characters that have proved to be highly variable, a fact that contributed to taxonomic inflation mostly in northern Africa. A detailed study and re-evaluation of morphological characters, together with recent phylogenetic data based on concatenation of two plastid and one nuclear region sequence data, yielded the basis for a taxonomic reappraisal of the series. In this respect, a new comprehensive integrative taxonomic arrangement for Biscutella ser. Biscutella is presented in which 10 taxa are accepted, namely seven species and three additional varieties. The name B. eriocarpa DC. is reinterpreted and suggested to include the highest morphological variation found in northern Morocco. Its treatment here accepts two varieties, one of which is described as new (B. eriocarpa var. riphaea A. Vicente, M. A.´ Alonso & M. B. Crespo). In addition, the circumscriptions of several species, such as B. boetica Boiss. & Reut., B. didyma L., B. lyrata L., and B. maritima Ten., are revisited. Nomenclatural types, synonymy, brief descriptions, cytogenetic data, conservation status, distribution maps, and identification keys are included for the accepted taxa, with seven lectotypes and one epitype being designated here. -

Cavers Et Al. 2003
Molecular Ecology (2003) 12, 1451–1460 doi: 10.1046/j.1365-294X.2003.01810.x ChloroplastBlackwell Publishing Ltd. DNA phylogeography reveals colonization history of a Neotropical tree, Cedrela odorata L., in Mesoamerica S. CAVERS,* C. NAVARRO† and A. J. LOWE* *Centre for Ecology and Hydrology-Edinburgh, Bush Estate, Penicuik, Midlothian EH26 0QB, UK, †Centro Agrónomico Tropical de Investigación y Enseñanza, Cartago, Turrialba 7170, Costa Rica Abstract Spanish Cedar (Cedrela odorata L.) is a globally important timber species which has been severely exploited in Mesoamerica for over 200 years. Using polymerase chain reaction– restriction fragment length polymorphisms, its chloroplast (cp) DNA phylogeography was studied in Mesoamerica with samples from 29 populations in six countries. Five haplotypes were characterized, phylogenetically grouped into three lineages (Northern, Central and Southern). Spatial analysis of ordered genetic distance confirmed deviation from a pattern of isolation by distance. The geographically proximate Northern and Central cpDNA lineages were genetically the most differentiated, with the Southern lineage appearing between them on a minimum spanning tree. However, populations possessing Southern lineage haplotypes occupy distinct moist habitats, in contrast to populations possessing Northern and Central lineage haplotypes which occupy drier and more seasonal habitats. Given the known colonization of the proto-Mesoamerican peninsula by South American flora and fauna prior to the formation of the Isthmus of Panama, it seems most likely that the observed population structure in C. odorata results from repeated colonization of Mesoamerica from South American source populations. Such a model would imply an ancient, pre-Isthmian colonization of a dry-adapted type (possessing the Northern lineage or a prototype thereof), with a secondary colonization via the land bridge. -

Ingredients Guide
INGREDIENTS LISTS Revive Skincare Products Body Wash Gentle: Aqua, Lauryl Glucoside (Coconut), Cocamidopropyl Betaine (Coconut), Sodium Lauroyl Sarcosinate (Coconut), Decyl Glucoside (Coconut), Coco Glucoside & Glyceryl Oleate, Isopropyl Myristate (Coconut), Guar Hydroxypropyltrimonium Chloride, Sodium Benzoate, Parfum (inc Essential Oils of Sweet Orange, Tangerine, Ginger, Geranium, Neroli and Jasmine), Argania Spinosa (Argan) Oil, Aloe Barbadensis (Aloe) Leaf Juice, Camellia Sinensis (Green Tea) Extract, Macadamia Ternifolia (Macadamia) Seed Oil, Oleoresin Rosmarinus Officinalis (Rosemary) Extract. Cleanser Foaming: Aqua, Cocamidopropyl Betaine (Coconut), Lauryl Glucoside (Coconut), Sodium Lauroyl Sarcosinate (Coconut), Sodium Benzoate, Coco Glucoside & Glyceryl Oleate, Isopropyl Myristate (Coconut), Guar Hydroxypropyltrimonium Chloride, Phenoxyethanol, Argania Spinosa (Argan) Oil, Aloe Barbadensis (Aloe) Leaf Juice, Camellia Sinensis (Green Tea) Extract, Macadamia Ternifolia (Macadamia) Seed Oil, Tocopheryl (Vitamin E), Citric Acid. Cleanser Cream : Aqua, Glycerin, Isopropyl Myristate (Coconut), Cocamidopropyl Betaine (Coconut), Glyceryl Stearate & PEG-100 Stearate (Coconut), Amygdalus Dulcis (Sweet Almond) Oil, Argania Spinosa (Argan) Oil, Cetearyl Alcohol (Coconut), Phenoxyethanol, Dimethicone, Tocopheryl (Vitamin E), Rosa Canina (Rosehip) Fruit Oil, Aloe Barbadensis (Aloe) Leaf Juice, Camellia Sinensis (Green Tea) Extract, Carbomer 940, Sodium Benzoate, Citrus Aurantium Dulcis (Sweet Orange) Extract, Oleoresin Rosmarinus Officinalis -
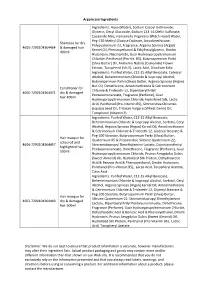
Arganicare Ingredients Disclaimer: We Do Our Best to Ensure That Product
Arganicare Ingredients Ingredients: Aqua (Water), Sodium Cocoyl Isethionate, Glycerin, Decyl Glucoside, Sodium C14-16 Olefin Sulfonate, Cocamide Mea, Hamamelis Virginiana (Witch Hazel) Water, Peg-120 Methyl Glucose Dioleate, Amodimethicone, Shampoo for dry Polyquaternium-22, Fragrance, Argania Spinosa (Argan) 4605-7290104364464 & damaged hair Kernel Oil, Phenoxyethanol & Ethylhexylglycerin, Dmdm 400ml Hydantoin, Niacinamide, Guar Hydroxypropyltrimonium Chloride, Panthenol (Pro-Vit. B5), Butyrospermum Parkii (Shea Butter) Oil, Anthemis Nobilis (Camomile) Flower Extract, Tocopherol (Vit.E), Lactic Acid, Disodium Edta Ingredients: Purifed Water, C12-15 Alkyl Benzoate, Cetearyl Alcohol, Behentrimonium Chloride & Isopropyl Alcohol, Butyrospermum Parkii (Shea) Butter, Argania Spinosa (Argan) Nut Oil, Dimethicone, Amodimethicone & Cetrimonium Conditioner for Chloride & Trideceth-12, Dipentaerythrityl 4606-7290104364471 dry & damaged Pentaisononanoate, Fragrance (Perfume), Guar hair 400ml Hydroxypropyltrimonium Chloride, Hydrolized Silk, Lactic Acid, Panthenol (Pro-Vitamin B5), Simmondsia Chinensis (Jojoba) Seed Oil, Triticum Vulgare (Wheat Germ) Oil, Tocopherol (Vitamin E) Ingredients: Purifed Water, C12-15 Alkyl Benzoate, Behentrimonium Chloride & Isopropyl Alcohol, Sorbitol, Cetyl Alcohol, Argania Spinosa (Argan) Kernel Oil, Amodimethicone & Cetrimonium Chloride & Trideceth-12, Glyceryl Stearate & Peg-100 Stearate, Butyrospermum Parkii (Shea) Butter, Hair masque for Quaternium-95 & Propanediol, Silicone Quaternium-22, coloured and 4604-7290104364457 -

Renoprotective, Hypoglycemic, and Hypolipidemic Effects of Argan (Argania Spinosa L.) Oil on Male Rats Exposed to Cadmium
ACTA SCIENTIFIC MEDICAL SCIENCES (ISSN: 2582-0931) Volume 4 Issue 2 February 2020 Research Article Renoprotective, Hypoglycemic, and Hypolipidemic Effects of Argan (Argania Spinosa L.) Oil on Male Rats Exposed to Cadmium Mujahid M Al-Muqati, Isam M Abu Zeid* and Naser A Al-Kenani Received: December 26, 2019 Department of Biological Sciences, Faculty of Sciences, King Abdulaziz University, Published: December 31, 2019 Saudi Arabia © All rights are reserved by Isam M Abu *Corresponding Author: Isam M Abu Zeid, Department of Biological Sciences, Zeid., et al. Faculty of Sciences, King Abdulaziz University, Saudi Arabia. DOI: 10.31080/ASMS.2020.04.0516 Abstract receiving considerable attention. The objective of the present study is to evaluate the protective effect of argan oil on male rats The protective and health benefits of various plant oils as renoprotective, hypoglycemic, and hypolipidemic agents are recently exposed to cadmium (Cd). In this study, 48 male rats were equally allocated into 4 groups. Rats of the first group (G1) served as normal controls. Rats of the second group (G2) were orally given 5mg/kg body weight cadmium chloride day after day. Rats the third group given to group two. Rats of the fourth group were orally supplemented with the same dose of argan oil given to group three. After six (G3) were orally administered with 700 mg/kg body weight argan oil and after three hours gave the same dose of cadmium chloride weeks of treatment, aassessments of fasting blood glucose, and serum urea nitrogen, uric acid, creatinine, low density lipoprotein- cholesterol (LDL-c) and high density lipoprotein-cholesterol (HDL-c) were performed in control and cadmium-intoxicated rats.