Prescribing Information Dipipanone 10Mg and Cyclizine 30Mg Tablets (Dipipanone Hydrochloride and Cyclizine Hydrochloride)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Best Bets from the Manchester Royal Infirmary Edited by K Mackway-Jones
247 BEST EVIDENCE TOPIC REPORTS Emerg Med J: first published as 10.1136/emj.19.3.248 on 1 May 2002. Downloaded from Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary Edited by K Mackway-Jones Clinical scenario Best evidence topic reports (BETs) summarise the evidence A 23 year old woman attends an emergency department hav- pertaining to particular clinical questions. They are not ing taken sixty 500 mg paracetamol tablets. Her four hour systematic reviews, but rather contain the best (highest paracetamol levels are above the treatment line. She does not level) evidence that can be practically obtained by busy want to be treated with intravenous therapy. You wonder practising clinicians. The search strategies used to find the whether oral antidote is as effective. best evidence are reported in detail in order to allow clinicians to update searches whenever necessary. The BETs published below were first reported at the Critical Three part question Appraisal Journal Club at the Manchester Royal Infirmary.1 In [patients who need an antidote for paracetamol overdose] Each BET has been constructed in the four stages that have is [intravenous therapy better than oral therapy] at [prevent- been described elsewhere.2 The BETs shown here together ing liver damage and death]? with those published previously and those currently under construction can be seen at http://www.bestbets.org.3 Six Search strategy BETs are included in this issue of the journal. Medline 1966 to 12/01 using the OVID interface. [exp acetyl- -

(12) Patent Application Publication (10) Pub. No.: US 2004/0024006 A1 Simon (43) Pub
US 2004.0024006A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0024006 A1 Simon (43) Pub. Date: Feb. 5, 2004 (54) OPIOID PHARMACEUTICAL May 30, 1997, now abandoned, and which is a COMPOSITIONS continuation-in-part of application No. 08/643,775, filed on May 6, 1996, now abandoned. (76) Inventor: David Lew Simon, Mansfield Center, CT (US) Publication Classification Correspondence Address: (51) Int. Cl. ................................................ A61K 31/485 David L. Simon (52) U.S. Cl. .............................................................. 514/282 P.O. Box 618 100 Cemetery Road (57) ABSTRACT Mansfield Center, CT 06250 (US) The invention is directed in part to dosage forms comprising a combination of an analgesically effective amount of an (21) Appl. No.: 10/628,089 opioid agonist analgesic and a neutral receptor binding agent or a partial mu-opioid agonist, the neutral receptor binding (22) Filed: Jul. 25, 2003 agent or partial mu-opioid agonist being included in a ratio Related U.S. Application Data to the opioid agonist analgesic to provide a combination product which is analgesically effective when the combina (63) Continuation-in-part of application No. 10/306,657, tion is administered as prescribed, but which is leSS analge filed on Nov. 27, 2002, which is a continuation-in-part Sically effective or less rewarding when administered in of application No. 09/922,873, filed on Aug. 6, 2001, excess of prescription. Preferably, the combination product now Pat. No. 6,569,866, which is a continuation-in affects an opioid dependent individual differently from an part of application No. 09/152,834, filed on Sep. -

Outline for Controlled Substances Program
Environmental Health and Safety Controlled Substances Program Date of Issuance: Review Date: 10/1/2019 (no changes) 10/01/2018 Revision Number: Initial Prepared by: EH&S Table of Contents HEADINGS Introduction Applicability Responsibilities Registration Requirements Authorized Use Ordering/Purchasing Administering and Dispensing Inventory Procedures (Continuing Records) Security Disposal FORMS: Registering or renewing a DEA or state license (CMU) Controlled Substances Authorized users list (CMU) Employee questionnaire for those with access to controlled substances (CMU) Record of Form 222 use (Order form) (CMU) Records of Controlled Substance Purchases (CMU) Record of Controlled Substance Administering and dispensing (CMU) Controlled Substance Physical Inventory (CMU) DEA Registration of Persons doing research or analysis (Form 225) DEA Registration of Dispensers (Form 224) DEA Registration Instructional (Form 224 and 226 to renew) DEA Report of loss or theft (Form 106) DEA Report of drugs surrendered (From 41) DEA SCHEDULES: Schedule I Schedule II Schedule III Schedule IV Schedule V INTRODUCTION State and Federal regulations have been promulgated concerning the use and handling of US Department of Justice Drug Enforcement Administration (DEA) controlled substances. These regulations are in place to address materials which are or have the potential to be addictive or habit forming. These substances have been categorized into “schedules” that have been created by the DEA to reflect their level of concern. The “Carnegie Mellon University DEA Controlled Substances Program” is intended to ensure that Carnegie Mellon University is in compliance with our regulatory requirements. Required activities under the DEA include: 1. Registration of your work with the DEA and with Carnegie Mellon’s Department of Environmental Health and Safety (EH&S). -

The Prevention and Treatment of Drug Misuse in Britain
If you have issues viewing or accessing this file contact us at NCJRS.gov. .. " FW The prevention and treatment of drug misuse in Britain "I Issued by Rererence Division BRITISH INFORMATION SERVICES All Agency of the British Government 845 THIRD AVENUE, NEW YORK, N.Y. ]0022 This material is prepared, edited, issued or circulated by British Information Servic(!s, 845 Third Avenue, New York, N. Y.l0022, which is registered under the Foreign Agents Registration Act as an agent of the Brilish Government. This material is filed with the Department of Justice where the required registration statement is available for public inspection. Registration does not indicate approval 0/ the contents of this material by the United States Government. PRINTED IN ENGLAND BY TRADE UNION LABOR BY COLUNS AND WILSON LTD., ANDOVER The prevention and treatment of drug misuse in Britain Prepared by REFERENCE DIVISION CENTRhL OFFICE OF INFORMATION, LONDON Oclo~'er 1978 Quote No RS94S/78 CLASSIFICATION 4(c) N.B.-This pamphlet is i//tellded to be used for referellce purposes alld may be freely used ill preparing articles, speeches, broadcasts, elc. No acknowledgment is necessary. Please Ilote the date ofpreparation. Tire text gives general gUidance only, and should 1I0t be treated as an authoritative statement of the law. Pamphlets ill this series may be obtained from the Tllformation Office at tlte British Embassy, Consulate or High Commission in tile inquirer's Co/lIltl'Y of residellce. CONTENTS Page INTRODUCTION 1 BACKGROUND .. 3 PREVENTING DRUG MISUSE 8 The Law 8 Health Education 17 TREATMENT AND REHABILITATION .. 20 Narcotic Drug Misuse and the Role of the Clinics 20 Treating Other Forms of Drug Misuse 26 Rehabilitation 26 INFORMATION AND RESEARCH ., 29 APPENDICES 1. -

Prescription of Controlled Drugs to Addicts
1876 BRITISH MEDICAL JOURNAL VOLUME 286 11 JUNE 1983 Br Med J (Clin Res Ed): first published as 10.1136/bmj.286.6381.1876 on 11 June 1983. Downloaded from For Debate . P Unacceptable face of private practice: prescription of controlled drugs to addicts THOMAS BEWLEY, A HAMID GHODSE Abstract practitioners. In the past three years, however, the pattern has shown a pronounced change (table), large numbers of addicts Self administered questionnaires completed by 69 out again having drugs prescribed for them by private general of 100 consecutive drug addicts two attending drug practitioners. These drugs are no longer heroin and cocaine dependence clinics that some suggested private general but other controlled drugs which are not included in the practitioners were easily persuaded to prescribe con- licensing regulations. trolled drugs. These drugs were usually methadone, dipipanone-cyclizine (Diconal), and methylphenidate (Ritalin). Numbers of new narcotic addicts notified to New narcotic addicts notified to Home Office the Home Office confirmed the practice, which may lead Source of notification to a severe spread of addiction, as occurred in the 1960s with heroin and cocaine. Year Hospital treatment Prison medical General medical Total centret officert practitionert If the General Medical Council or a tribunal set up 1972 480 250 80 800 in accordance with the Misuse of Drugs Act 1971 cannot 1973 430 230 150 807 stop then the 1974 380 240 250 870 the practice, present licensing system 1975 390 270 260 922 should be extended to include all controlled drugs. 1976 400 290 290 984 1977 450 290 370 1109 1978 590 240 520 1347 1979 610 270 720 1597 1980 570 240 790 1600 Introduction 1981* 760 320 1220 2300 Prescribing drugs of addiction to addicts is accepted medical Provisional. -

Alternative Opioids to Morphine in Palliative Care: Postgrad Med J: First Published As 10.1136/Pmj.77.908.371 on 1 June 2001
Postgrad Med J 2001;77:371–378 371 Alternative opioids to morphine in palliative care: Postgrad Med J: first published as 10.1136/pmj.77.908.371 on 1 June 2001. Downloaded from a review of current practice and evidence M Barnett This is a review of current practice of opioid x Nausea/vomiting use in palliative care, conducted from the per- x Urinary retention spective of a practising clinician working in the x Myoclonus increasingly complex area of symptom control. x Paradoxical pain In examining alternative opioids to morphine, x Respiratory depression choice and availability of diVerent drugs reflect For practical purposes, the most important the UK perspective. Some drugs or formula- side eVects are sedation, nausea, and constipa- tions may not be available elsewhere, but the tion. principles discussed may hopefully still be Sedation and nausea occur particularly when applied. starting the drug, usually temporarily, but may The aims of this paper are several-fold: recur with dose increases. Nausea can be (1) To present an overview of available opio- pre-empted by using a centrally acting an- ids. tiemetic. This is not always necessary but (2) To consider factors aVecting possible advisable if the patient is already nauseated or choice of opioid—with particular reference fearful about it. Sedation is usually unavoidable to the palliative care setting. but short lived (48–72 hours) among patients (3) To consider availability and limitations of starting oV on low doses. It may become more current data which may aVect evidence intractable at high dose, and there is some work based decisions. -

Disposal Systems of Transdermal Delivery Devices to Prevent Misuse of the Active Agents Contained Therein
(19) & (11) EP 1 837 023 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 26.09.2007 Bulletin 2007/39 A61K 31/485 (2006.01) A61K 9/70 (2006.01) A61K 31/445 (2006.01) B09B 3/00 (2006.01) (2006.01) (21) Application number: 07003694.2 A61F 13/00 (22) Date of filing: 10.06.2003 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Baker, Carl J. HU IE IT LI LU MC NL PT RO SE SI SK TR Middletown, NY 10941-5004 (US) Designated Extension States: • Shevchuck, Ihor AL LT LV MK Yonkers, NY 10710 (US) (30) Priority: 10.06.2002 US 387800 P (74) Representative: Maiwald Patentanwalts GmbH Elisenhof, (62) Document number(s) of the earlier application(s) in Elisenstrasse 3 accordance with Art. 76 EPC: 80335 München (DE) 03757468.8 / 1 513 532 Remarks: (71) Applicant: EURO-CELTIQUE S.A. This application was filed on 22 - 02 - 2007 as a 2330 Luxembourg (LU) divisional application to the application mentioned under INID code 62. (54) Disposal systems of transdermal delivery devices to prevent misuse of the active agents contained therein (57) The present invention relates to a transdermal at least one deactivating agent to chemically alter, to de- delivery device disposal system for disposing of a grade, and/or to deactivate the active component (s) con- transdermal delivery device containing at least one phar- tained in the transdermal delivery device, such as an opi- maceutically active component. The disposal system oid antagonist or an opioid agonist deactivating agent so contains -

ESTIMATED WORLD REQUIREMENTS of NARCOTIC DRUGS in GRAMS for 2013 (January Update)
ESTIMATED WORLD REQUIREMENTS OF NARCOTIC DRUGS IN GRAMS FOR 2013 (January update) Afghanistan Oxycodone 43 000 Codeine 50 000 Oxymorphone 300 Dextropropoxyphene 2 000 000 Pethidine 65 000 Diphenoxylate 20 000 Remifentanil 9 100 Fentanyl 6 Sufentanil 1 Methadone 6 000 Thebaine 45 000 Morphine 4 000 Armenia Pethidine 80 000 Codeine 3 000 Pholcodine 100 000 Fentanyl 21 Albania Methadone 10 000 Codeine 35 000 Morphine 4 500 Fentanyl 40 Thebaine 10 Methadone 9 000 Trimeperidine 650 Morphine 3 000 Aruba* Pethidine 2 500 Alfentanil 3 Pholcodine 1 000 Bezitramide 1 Remifentanil 8 Cocaine 70 Sufentanil 1 Codeine 85 Algeria Dextromoramide 1 Alfentanil 500 Dextropropoxyphene 85 Codeine 1 000 000 Fentanyl 130 Etorphine 1 Hydrocodone 2 Fentanyl 1 000 Methadone 150 Morphine 11 000 Morphine 340 Pethidine 3 000 Opium 450 Pholcodine 2 500 000 Oxycodone 26 Sufentanil 30 Pethidine 404 Andorra Piritramide 20 Fentanyl 80 Remifentanil 19 Methadone 1 000 Ascension Island Morphine 500 Alfentanil 1 Oxycodone 1 500 Fentanyl 1 Pethidine 500 Morphine 2 Remifentanil 4 Pethidine 9 Angola* Australia Alfentanil 2 Alfentanil 400 Codeine 30 000 Cannabis 21 500 Dextromoramide 375 Cocaine 20 000 Dihydrocodeine 375 Codeine 9 800 000 Fentanyl 45 Conc. of poppy straw Morphine 11 000 AOA 4 000 000 Pethidine 13 000 ATA 85 000 000 Sufentanil 2 Dextromoramide 10 Anguilla Dextropropoxyphene 1 925 000 Fentanyl 1 Difenoxin 7 Morphine 20 Dihydrocodeine 285 000 Pethidine 300 Diphenoxylate 80 000 Antigua and Barbuda* Ethylmorphine 10 Cocaine 9 Etorphine 2 Codeine 169 Fentanyl 40 000 -

English → French → English
Anglais → Français - A - Acetorphine Acétorphine Acetorphine hydrochloride Chlorhydrate d’acétorphine Acetyl-alpha-methylfentanyl Acétyl-alpha-méthylfentanyl Acetyldihydrocodeine Acétyldihydrocodéine Acetyldihydrocodeine hydrochloride Chlorhydrate d’acétyldihydrocodéine Acetylmethadol Acétylméthadol Alfentanil Alfentanil Alfentanil hydrochloride Chlorhydrate d’alfentanil Allobarbital Allobarbital Allobarbital-aminophenazone Allobarbital-aminophénazone Allylprodine Allylprodine Allylprodine hydrochloride Chlorhydrate d’allylprodine Alphacetylmethadol Alphacétylméthadol Alphacetylmethadol hydrochloride Chlorhydrate d’alphacétylméthadol Alphameprodine Alphaméprodine Alphamethadol Alphaméthadol Alpha-methylfentanyl Alpha-méthylfentanyl Alpha-methylfentanyl hydrochloride Chlorhydrate d’alpha-méthylfentanyl Alpha-methylthiofentanyl Alpha-méthylthiofentanyl Alpha-methylthiofentanyl hydrochloride Chlorhydrate d’alpha-méthylthiofentanyl Alphaprodine Alphaprodine Alphaprodine hydrochloride Chlorhydrate d’alphaprodine Alprazolam Alprazolam Amfepramone Amfépramone Amfepramone glutamate Glutamate d’amfépramone Amfepramone hydrochloride Chlorhydrate d’amfépramone Amfepramone resinate Amfépramone résinate Amfetamine Amfétamine Amfetamine acetylsalicylate Acétylsalicylate d’amfétamine Amfetamine adipate Adipate d’amfétamine Amfetamine p-aminophenylacetate Acétate p-aminophényle d’amfétamine Amfetamine aspartate Aspartate d’amfétamine Amfetamine (4-chlorophenoxy) acetate Acétate (chlorophénoxy-4) d’amfétamine Amfetamine hydrochloride Chlorhydrate d’amfétamine -
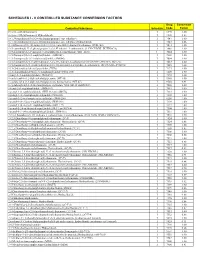
Controlled Substance Conversion Factors
SCHEDULES I - V CONTROLLED SUBSTANCE CONVERSION FACTORS Drug Conversion Controlled Substance Schedule Code Factor (+/-)cis -4-Methylaminorex I 1590 1.00 (+/-)cis -4-Methylaminorex Hydrochloride I 1590 0.83 1-(1,3-benzodioxol-5-yl)-2-(ethylamino)propan-1-one (ethylone) I 7547 1.00 1-(1,3-benzodioxol-5-yl)-2-(ethylamino)propan-1-one (ethylone) hydrochloride I 7547 0.86 (1-(4-fluorobenzyl)-1H -indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone (FUB-144) I 7014 1.00 1-(4-cyanobutyl)-N -(2-phenylpropan-2-yl)-1H -indazole-3-carboxamide (4-CN-CUMYL-BUTINACA) I 7089 1.00 1-(5-fluoropentyl)-1H -indazol-3-yl](naphthalen-1-yl)methanone (THJ–2201) 1 7024 1.00 1-(5-fluoropentyl)-3-(1-naphthoyl)indole (AM2201) I 7201 1.00 1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole (AM694) I 7694 1.00 1-(5-fluoropentyl)-N -(2-phenylpropan-2-yl)-1H -indazole-3-carboxamide (5F-CUMYL-PINACA; SGT-25) I 7083 1.00 1-(5-fluoropentyl)-N -(2-phenylpropan-2-yl)-1H -pyrrolo[2,3-b]pyridine-3-carboxamide (5F-CUMYL-P7AICA) I 7085 1.00 1-[1-(2-thienyl)cyclohexyl]pyrrolidine (TCPy) I 7473 1.00 1-[2-(4-morpholinyl)ethyl]-3-(1-naphthoyl)indole (JWH- 200) I 7200 1.00 1-butyl-3-(1-naphthoyl)indole (JWH-073) I 7173 1.00 1-cyclohexyl-4-(1,2-diphenylethyl)piperazine (MT-45) I 9560 1.00 1-cyclohexyl-4-(1,2-diphenylethyl)piperazine hydrochloride (MT-45) I 9560 0.91 1-cyclohexylethyl-3-(2-methoxyphenylacetyl)indole 7008 (SR-18 and RCS-8) I 7008 1.00 1-hexyl-3-(1-naphthoyl)indole (JWH-019) I 7019 1.00 1-pentyl-3-(1-naphthoyl)indole (JWH-018 and AM678) I 7118 1.00 1-pentyl-3-(2-chlorophenylacetyl)indole -

Formulary Book : Contents NHS Canterbury and Coastal CCG
Formulary Book : Contents NHS Canterbury And Coastal CCG Subparagraph Page Nbr. 1.1.2.1 Gastro-intestinal system | Dyspep&Gastro-Oesophageal Reflux Disease | Other Drugs for Dyspepsia and GORD 8 1.2 Gastro-intestinal system | Antispasmod.&Other Drgs Alt.Gut Motility 9 1.3.1 Gastro-intestinal system | Antisecretory Drugs+Mucosal Protectants | H2-Receptor Antagonists 10 1.3.5 Gastro-intestinal system | Antisecretory Drugs+Mucosal Protectants | Proton Pump Inhibitors 10 1.4.2 Gastro-intestinal system | Acute Diarrhoea | Antimotility Drugs 13 1.4.3 Gastro-intestinal system | Acute Diarrhoea | Enkephalinase Inhibitors 13 1.5 Gastro-intestinal system | Chronic Bowel Disorders 13 1.5.1 Gastro-intestinal system | Chronic Bowel Disorders | Aminosalicylates 13 1.5.2 Gastro-intestinal system | Chronic Bowel Disorders | Corticosteroids 16 1.6.2 Gastro-intestinal system | Laxatives | Stimulant Laxatives 16 1.6.4 Gastro-intestinal system | Laxatives | Osmotic Laxatives 17 1.6.6 Gastro-intestinal system | Laxatives | Peripheral opioid receptor antagonists 18 1.6.7 Gastro-intestinal system | Laxatives | 5HT4-receptor agonists 18 1.7.2 Gastro-intestinal system | Local Prepn for Anal & Rectal Disorders | Co Haemorrhoidal Prep's + Corticosteroid 19 1.7.4 Gastro-intestinal system | Local Prepn for Anal & Rectal Disorders | Management of Anal Fissures 19 1.9.1 Gastro-intestinal system | Drugs Affecting Intestinal Secretions | Drugs Affecting Biliary Composition&Flow 19 2.1.1 Cardiovascular system | Positive Inotropic Drugs | Cardiac Glycosides 19 2.1.2 Cardiovascular -

PDF (The Abuse of Prescribed Medication)
PSYCHIATRY The abuse of prescribed medication JOHN J. O’CONNOR & SALLY STAFFORD-JOHNSON The prevalence and management of prescribed Amphetamines are synthetic stimulants first produced in 1887, medication abuse is outlined. The patterns of but not used medically until the 1930’s. During the Second World presentation are related to availability and the abuser’s War and the Vietnam War profile. Ireland recorded a world first in the abuse of amphetamines (Dexedrine) were used to increase the performance of Diconal. Methadone is replacing heroin as a problem soldiers. In the 1950’s and 1960’s drug among abusers. Early recognition and intervention they were widely prescribed as slimming tablets and used to treat give good treatment outcomes. mild depression. The non medical use of amphetamines was very popular among teenagers during the 1960’s when large quantities of “purple hearts” were taken to stay awake at parties. A number of other drugs such as methylphenidate (Ritalin), diehylpropin hydrochlor. (Tenutate Dospan) and fenfluramine hydrochlor. (Ponderax) have amphetamine like effects; the latter two are usually used as sliming agents while methylphenidate is mainly used in the treatment of narcolepsy and the hyperkinetic syndrome. Tolerance to many of the effects of amphetamines develops rapidly, with some users taking up to 1 gramme per day. When the drug is discontinued a user feels depressed, fatigued, sleepy and extremely hungry. The amphe- tamine has _____________________________ John J. O’Connor, MRCPsych, Consultant Psychiatrist/Director of Clinical Programmes, Sally Stafford- Johnson, MA, Senior Clinical Psychologist, Drug Treatment Centre, Trinity Court, 30-31 Pearse Street, Dublin 2. This article is a reproduction of that published in: Irish Doctor, March 1990, pp.217-221.