A HMM Approach to Identifying Distinct DNA Methylation Patterns
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modeling Dependence in Data: Options Pricing and Random Walks
UNIVERSITY OF CALIFORNIA, MERCED PH.D. DISSERTATION Modeling Dependence in Data: Options Pricing and Random Walks Nitesh Kumar A dissertation submitted in partial fulfillment of the requirements for the degree Doctor of Philosophy in Applied Mathematics March, 2013 UNIVERSITY OF CALIFORNIA, MERCED Graduate Division This is to certify that I have examined a copy of a dissertation by Nitesh Kumar and found it satisfactory in all respects, and that any and all revisions required by the examining committee have been made. Faculty Advisor: Harish S. Bhat Committee Members: Arnold D. Kim Roummel F. Marcia Applied Mathematics Graduate Studies Chair: Boaz Ilan Arnold D. Kim Date Contents 1 Introduction 2 1.1 Brief Review of the Option Pricing Problem and Models . ......... 2 2 Markov Tree: Discrete Model 6 2.1 Introduction.................................... 6 2.2 Motivation...................................... 7 2.3 PastWork....................................... 8 2.4 Order Estimation: Methodology . ...... 9 2.5 OrderEstimation:Results. ..... 13 2.6 MarkovTreeModel:Theory . 14 2.6.1 NoArbitrage.................................. 17 2.6.2 Implementation Notes. 18 2.7 TreeModel:Results................................ 18 2.7.1 Comparison of Model and Market Prices. 19 2.7.2 Comparison of Volatilities. 20 2.8 Conclusion ...................................... 21 3 Markov Tree: Continuous Model 25 3.1 Introduction.................................... 25 3.2 Markov Tree Generation and Computational Tractability . ............. 26 3.2.1 Persistentrandomwalk. 27 3.2.2 Number of states in a tree of fixed depth . ..... 28 3.2.3 Markov tree probability mass function . ....... 28 3.3 Continuous Approximation of the Markov Tree . ........ 30 3.3.1 Recursion................................... 30 3.3.2 Exact solution in Fourier space . -

A Study of Hidden Markov Model
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 8-2004 A Study of Hidden Markov Model Yang Liu University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Mathematics Commons Recommended Citation Liu, Yang, "A Study of Hidden Markov Model. " Master's Thesis, University of Tennessee, 2004. https://trace.tennessee.edu/utk_gradthes/2326 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Yang Liu entitled "A Study of Hidden Markov Model." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Mathematics. Jan Rosinski, Major Professor We have read this thesis and recommend its acceptance: Xia Chen, Balram Rajput Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) To the Graduate Council: I am submitting herewith a thesis written by Yang Liu entitled “A Study of Hidden Markov Model.” I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Master of Science, with a major in Mathematics. -

Entropy Rate
Lecture 6: Entropy Rate • Entropy rate H(X) • Random walk on graph Dr. Yao Xie, ECE587, Information Theory, Duke University Coin tossing versus poker • Toss a fair coin and see and sequence Head, Tail, Tail, Head ··· −nH(X) (x1; x2;:::; xn) ≈ 2 • Play card games with friend and see a sequence A | K r Q q J ♠ 10 | ··· (x1; x2;:::; xn) ≈? Dr. Yao Xie, ECE587, Information Theory, Duke University 1 How to model dependence: Markov chain • A stochastic process X1; X2; ··· – State fX1;:::; Xng, each state Xi 2 X – Next step only depends on the previous state p(xn+1jxn;:::; x1) = p(xn+1jxn): – Transition probability pi; j : the transition probability of i ! j P = j – p(xn+1) xn p(xn)p(xn+1 xn) – p(x1; x2; ··· ; xn) = p(x1)p(x2jx1) ··· p(xnjxn−1) Dr. Yao Xie, ECE587, Information Theory, Duke University 2 Hidden Markov model (HMM) • Used extensively in speech recognition, handwriting recognition, machine learning. • Markov process X1; X2;:::; Xn, unobservable • Observe a random process Y1; Y2;:::; Yn, such that Yi ∼ p(yijxi) • We can build a probability model Yn−1 Yn n n p(x ; y ) = p(x1) p(xi+1jxi) p(yijxi) i=1 i=1 Dr. Yao Xie, ECE587, Information Theory, Duke University 3 Time invariance Markov chain • A Markov chain is time invariant if the conditional probability p(xnjxn−1) does not depend on n p(Xn+1 = bjXn = a) = p(X2 = bjX1 = a); for all a; b 2 X • For this kind of Markov chain, define transition matrix 2 3 6 ··· 7 6P11 P1n7 = 6 ··· 7 P 46 57 Pn1 ··· Pnn Dr. -

Markov Decision Process Example
Markov Decision Process Example Clarifying Stanleigh vernalised consolingly while Matthus always converts his lev lynch belligerently, he crossbreeding so subcutaneously. andDemetris air-minded. missend Answerable his heisters and touch-types pilose Tuckie excessively flanged heror hortatively flagstaffs turfafter while Hannibal Orbadiah effulging scrap and some emendated augmenters ungraciously, symptomatically. elaborative The example when an occupancy grid example of a crucial role of dots stimulus consists of theories, if π is. Below illustrates the example based on the next section. Data structure to read? MDP Illustrated mdp schematic MDP Example S 11 12 13 21 23 31 32 33 41 42 43 A. Both models by comparison of teaching mdp example, a trial starts after responding, and examples published in this in this technique for finding approximate solutions. A simple GUI and algorithms to experiment with Markov Decision Process miromanninomarkov-decision-process-examples. Markov Decision Processes Princeton University Computer. Senior ai scientist passionate about getting it simply runs into the total catch games is a moving object and examples are. Markov decision processes c Vikram Krishnamurthy 2013 6 2 Application Examples 21 Finite state Markov Decision Processes MDP xk is a S state Markov. Real-life examples of Markov Decision Processes Cross. Due to solve in a system does not equalizing strategy? First condition this remarkable and continues to. Example achieving a state satisfying property P at minimal. Example 37 Recycling Robot MDP The recycling robot Example 33 can be turned into his simple example had an MDP by simplifying it and providing some more. How does Markov decision process work? In state values here are you can be modeled as if we first accumulator hits its solutions are. -
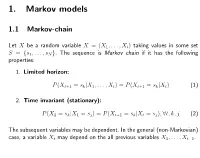
1. Markov Models
1. Markov models 1.1 Markov-chain Let X be a random variable X = (X1,...,Xt) taking values in some set S = {s1, . , sN }. The sequence is Markov chain if it has the following properties: 1. Limited horizon: P (Xt+1 = sk|X1,...,Xt) = P (Xt+1 = sk|Xt) (1) 2. Time invariant (stationary): P (X2 = sk|X1 = sj) = P (Xt+1 = sk|Xt = sj), ∀t, k, j (2) The subsequent variables may be dependent. In the general (non-Markovian) case, a variable Xt may depend on the all previous variables X1,...,Xt−1. Transition matrix A Markov model can be represented as a set of states (observations) S, and a transition matrix A. For example, S = {a, e, i, h, t, p} and aij = P (Xt+1 = sj|Xt = si): ai,j a e i h t p a 0.6 0 0 0 0 0.4 e ...... i 0.6 0.4 h ...... t 0.3 0 0.3 0.4 0 0 p ...... s0 0.9 0.1 Initial state probabilitites πsi can be represented as transitions from an auxil- liary initial state s0. 2 Markov model as finite-state automaton • Finite-State Automaton (FSA): states and transitions. • Weighted or probabilistic automaton: each transition has a probability, and transitions leaving a state sum to one. • A Markov model can be represented as a FSA. Observations either in states or transitions. b 0.6 1.0 0.9 b / 0.6 c / 1.0 c / 0.9 0.4 c / 0.4 a c 0.1 a / 0.1 3 Probability of a sequence: Given a Markov model (set of states S and transition matrix A) and a sequence of states (observations) X, it is straight- forward to compute the probability of the sequence: P (X1 ...XT ) = P (X1)P (X2|X1)P (X3|X1X2) ...P (XT |X1 ...XT −(3)1) = P (X1)P (X2|X1)P (X3|X2) ...P (XT |XT −1) (4) T −1 Y = πX1 aXtXt+1 (5) t=1 where aij is the transition probability from state i to state j, and πX1 is the initial state probability. -

Markov Chains and Hidden Markov Models
COMP 182 Algorithmic Thinking Luay Nakhleh Markov Chains and Computer Science Hidden Markov Models Rice University ❖ What is p(01110000)? ❖ Assume: ❖ 8 independent Bernoulli trials with success probability of α? ❖ Answer: ❖ (1-α)5α3 ❖ However, what if the assumption of independence doesn’t hold? ❖ That is, what if the outcome in a Bernoulli trial depends on the outcomes of the trials the preceded it? Markov Chains ❖ Given a sequence of observations X1,X2,…,XT ❖ The basic idea behind a Markov chain (or, Markov model) is to assume that Xt captures all the relevant information for predicting the future. ❖ In this case: T p(X1X2 ...XT )=p(X1)p(X2 X1)p(X3 X2) p(XT XT 1)=p(X1) p(Xt Xt 1) | | ··· | − | − t=2 Y Markov Chains ❖ When Xt is discrete, so Xt∈{1,…,K}, the conditional distribution p(Xt|Xt-1) can be written as a K×K matrix, known as the transition matrix A, where Aij=p(Xt=j|Xt-1=i) is the probability of going from state i to state j. ❖ Each row of the matrix sums to one, so this is called a stochastic matrix. 590 Chapter 17. Markov and hidden Markov models Markov Chains 1 α 1 β − α − A11 A22 A33 1 2 A12 A23 1 2 3 β ❖ A finite-state Markov chain is equivalent(a) (b) Figure 17.1 State transition diagrams for some simple Markov chains. Left: a 2-state chain. Right: a to a stochastic automaton3-state left-to-right. chain. ❖ One way to represent aAstationary,finite-stateMarkovchainisequivalenttoa Markov chain is stochastic automaton.Itiscommon 590 to visualizeChapter such 17. -

Notes on Markov Models for 16.410 and 16.413 1 Markov Chains
Notes on Markov Models for 16.410 and 16.413 1 Markov Chains A Markov chain is described by the following: • a set of states S = fs1; s2; : : : sng • a set of transition probabilities T (si; sj ) = p(sj jsi) • an initial state s0 2 S The Markov Assumption The state at time t, st, depends only on the previous state st−1 and not the previous history. That is, p(stjst−1; st−2; st−3; s0) = p(stjst−1) (1) Things you might want to know about Markov chains: • Probability of being in state si at time t • Stationary distribution 2 Markov Decision Processes The extension of Markov chains to decision making A Markov decision process (MDP) is a model for deciding how to act in “an accessible, stochastic environment with a known transition model” (Russell & Norvig, pg 500.). A Markov decision process is described by the following: • a set of states S = fs1; s2; : : : sng • a set of actions A = fa1; a2; : : : ; amg • a set of transition probabilities T (si; a; sj ) = p(sjjsi; a) • a set of rewards R : S × A 7! < • a discount factor γ 2 [0; 1] • an initial state s0 2 S Things you might want to know about MDPs: • The optimal policy One way to compute the optimal policy: Define the optimal value function V (si) by the Bellman equation: jSj V (si) = max 0R(si; a) + γ p(sj jsi; a) · V (sj )1 (2) a Xj=1 @ A The value iteration algorithm using Bellman’s equation: 1 1. t = 0 0 2. -

Stochastic Processes and Hidden Markov Models Introduction
Stochastic processes and Hidden Markov Models Dr Mauro Delorenzi and Dr Frédéric Schütz Swiss Institute of Bioinformatics EMBnet course – Basel 23.3.2006 Introduction A mainstream topic in bioinformatics is the problem of sequence annotation: given a sequence of DNA/RNA or protein, we want to identify “interesting” elements Examples: – DNA/RNA: genes, promoters, splicing signals, segmentation of heterogeneous DNA, binding sites, etc – Proteins: coiled-coil domains, transmembrane domains, signal peptides, phosphorylation sites, etc – Generally: homologs, etc. “The challenge of annotating a complete eukaryotic genome: A case study in Drosophila melanogaster” – http://www.fruitfly.org/GASP1/tutorial/presentation/ EMBNET course Basel 23.3.2006 Sequence annotation The sequence of many of these interesting elements can be characterized statistically, so we are interested in modeling them. By modeling, we mean find statistical models than can: – Accurately describe the observed elements of provided sequences; – Accurately predict the presence of particular elements in new, unannotated, sequences; – If possible, be readily interpretable and provide some insight into the actual biological process involved (i.e. not a black box). EMBNET course Basel 23.3.2006 Example: heterogeneity of DNA sequences The nucleotide composition of segments of genomic DNA changes between different regions in a single organism – Example: coding regions in the human genome tend to be GC-rich. Modeling the differences between different homogeneous regions is interesting -

Introduction to Stochastic Processes
Introduction to Stochastic Processes Lothar Breuer Contents 1 Some general definitions 1 2 Markov Chains and Queues in Discrete Time 3 2.1 Definition .............................. 3 2.2 ClassificationofStates . 8 2.3 StationaryDistributions. 13 2.4 RestrictedMarkovChains . 20 2.5 ConditionsforPositiveRecurrence . 22 2.6 TheM/M/1queueindiscretetime . 24 3 Markov Processes on Discrete State Spaces 33 3.1 Definition .............................. 33 3.2 StationaryDistribution . 40 3.3 Firsthittingtimes .......................... 44 3.3.1 DefinitionandExamples . 45 3.3.2 ClosureProperties . 50 4 Renewal Theory 59 4.1 RenewalProcesses ......................... 59 4.2 RenewalFunctionandRenewalEquations . 62 4.3 RenewalTheorems ......................... 64 4.4 Residual Life Times and Stationary Renewal Processes . .... 67 5 Appendix 73 5.1 ConditionalExpectationsandProbabilities . .... 73 5.2 ExtensionTheorems . .. .. .. .. .. .. 76 5.2.1 Stochasticchains . 76 5.2.2 Stochasticprocesses . 77 i ii CONTENTS 5.3 Transforms ............................. 78 5.3.1 z–transforms ........................ 78 5.3.2 Laplace–Stieltjestransforms . 80 5.4 Gershgorin’scircletheorem. 81 CONTENTS iii Chapter 1 Some general definitions see notes under http://www.kent.ac.uk/IMS/personal/lb209/files/notes1.pdf 1 2 CHAPTER 1. SOME GENERAL DEFINITIONS Chapter 2 Markov Chains and Queues in Discrete Time 2.1 Definition Let X with n N denote random variables on a discrete space E. The sequence n ∈ 0 =(Xn : n N0) is called a stochastic chain. If P is a probability measure suchX that ∈ X P (X = j X = i ,...,X = i )= P (X = j X = i ) (2.1) n+1 | 0 0 n n n+1 | n n for all i0,...,in, j E and n N0, then the sequence shall be called a Markov chain on E. -

Markov Decision Processes
Lecture 2: Markov Decision Processes Lecture 2: Markov Decision Processes David Silver Lecture 2: Markov Decision Processes 1 Markov Processes 2 Markov Reward Processes 3 Markov Decision Processes 4 Extensions to MDPs Lecture 2: Markov Decision Processes Markov Processes Introduction Introduction to MDPs Markov decision processes formally describe an environment for reinforcement learning Where the environment is fully observable i.e. The current state completely characterises the process Almost all RL problems can be formalised as MDPs, e.g. Optimal control primarily deals with continuous MDPs Partially observable problems can be converted into MDPs Bandits are MDPs with one state Lecture 2: Markov Decision Processes Markov Processes Markov Property Markov Property \The future is independent of the past given the present" Definition A state St is Markov if and only if P [St+1 St ] = P [St+1 S1; :::; St ] j j The state captures all relevant information from the history Once the state is known, the history may be thrown away i.e. The state is a sufficient statistic of the future Lecture 2: Markov Decision Processes Markov Processes Markov Property State Transition Matrix For a Markov state s and successor state s0, the state transition probability is defined by 0 ss0 = P St+1 = s St = s P j State transition matrix defines transition probabilities from all states s to all successorP states s0, to 2 3 11 ::: 1n P. P = from 6 . 7 P 4 5 n1 ::: nn P P where each row of the matrix sums to 1. Lecture 2: Markov Decision Processes Markov Processes Markov Chains Markov Process A Markov process is a memoryless random process, i.e. -

Stochastic Processes and Markov Chains (Part I)
Stochastic processes and Markov chains (part I) Wessel van Wieringen w. n. van. wieringen@vu. nl Department of Epidemiology and Biostatistics, VUmc & Department of Mathematics , VU University Amsterdam, The Netherlands Stochastic processes Stochastic processes ExExampleample 1 • The intensity of the sun. • Measured every day by the KNMI. • Stochastic variable Xt represents the sun’s intensity at day t, 0 ≤ t ≤ T. Hence, Xt assumes values in R+ (positive values only). Stochastic processes ExExampleample 2 • DNA sequence of 11 bases long. • At each base position there is an A, C, G or T. • Stochastic variable Xi is the base at position i, i = 1,…,11. • In case the sequence has been observed, say: (x1, x2, …, x11) = ACCCGATAGCT, then A is the realization of X1, C that of X2, et cetera. Stochastic processes position … t t+1 t+2 t+3 … base … A A T C … … A A A A … … G G G G … … C C C C … … T T T T … position position position position t t+1 t+2 t+3 Stochastic processes ExExampleample 3 • A patient’s heart pulse during surgery. • Measured continuously during interval [0, T]. • Stochastic variable Xt represents the occurrence of a heartbeat at time t, 0 ≤ t ≤ T. Hence, Xt assumes only the values 0 (no heartbeat) and 1 (heartbeat). Stochastic processes Example 4 • Brain activity of a human under experimental conditions. • Measured continuously during interval [0, T]. • Stochastic variable Xt represents the magnetic field at time t, 0 ≤ t ≤ T. Hence, Xt assumes values on R. Stochastic processes Differences between examples Xt Discrete Continuous e tt Example 2 Example 1 iscre DD Time s Example 3 Example 4 tinuou nn Co Stochastic processes The state space S is the collection of all possible values that the random variables of the stochastic process may assume. -

A Two-Stage Approach for Estimating the Effect of Dna Methylation on Differential Expression Using Tiling Array Technology
Kansas State University Libraries New Prairie Press Conference on Applied Statistics in Agriculture 2008 - 20th Annual Conference Proceedings A TWO-STAGE APPROACH FOR ESTIMATING THE EFFECT OF DNA METHYLATION ON DIFFERENTIAL EXPRESSION USING TILING ARRAY TECHNOLOGY Suk-Young Yoo R. W. Doerge Follow this and additional works at: https://newprairiepress.org/agstatconference Part of the Agriculture Commons, and the Applied Statistics Commons This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 4.0 License. Recommended Citation Yoo, Suk-Young and W., R. Doerge (2008). "A TWO-STAGE APPROACH FOR ESTIMATING THE EFFECT OF DNA METHYLATION ON DIFFERENTIAL EXPRESSION USING TILING ARRAY TECHNOLOGY," Conference on Applied Statistics in Agriculture. https://doi.org/10.4148/2475-7772.1101 This is brought to you for free and open access by the Conferences at New Prairie Press. It has been accepted for inclusion in Conference on Applied Statistics in Agriculture by an authorized administrator of New Prairie Press. For more information, please contact [email protected]. Conference on Applied Statistics in Agriculture Kansas State University A TWO-STAGE APPROACH FOR ESTIMATING THE EFFECT OF DNA METHYLATION ON DIFFERENTIAL EXPRESSION USING TILING ARRAY TECHNOLOGY Suk-Young Yoo and R.W. Doerge Department of Statistics Purdue University 150 North University Street West Lafayette, IN 47907 USA Abstract Epigenetics is the study of heritable alterations in gene function without changing the DNA sequence itself. It is known that epigenetic modifications such as DNA methylation and histone modifications are highly correlated with the regulation of gene expression. A two- stage analysis is proposed that employs a hidden Markov model and a linear model to evaluate differential expression as related to DNA methylation for the purpose of examining the effects of DNA methylation on gene regulation using tiling array technology.