A Two-Stage Approach for Estimating the Effect of Dna Methylation on Differential Expression Using Tiling Array Technology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modulation of Mrna and Lncrna Expression Dynamics by the Set2–Rpd3s Pathway
ARTICLE Received 20 Apr 2016 | Accepted 7 Oct 2016 | Published 28 Nov 2016 DOI: 10.1038/ncomms13534 OPEN Modulation of mRNA and lncRNA expression dynamics by the Set2–Rpd3S pathway Ji Hyun Kim1,2,*, Bo Bae Lee1,2,*, Young Mi Oh1,2, Chenchen Zhu3,4,5, Lars M. Steinmetz3,4,5, Yookyeong Lee1, Wan Kyu Kim1, Sung Bae Lee6, Stephen Buratowski7 & TaeSoo Kim1,2 H3K36 methylation by Set2 targets Rpd3S histone deacetylase to transcribed regions of mRNA genes, repressing internal cryptic promoters and slowing elongation. Here we explore the function of this pathway by analysing transcription in yeast undergoing a series of carbon source shifts. Approximately 80 mRNA genes show increased induction upon SET2 deletion. A majority of these promoters have overlapping lncRNA transcription that targets H3K36me3 and deacetylation by Rpd3S to the mRNA promoter. We previously reported a similar mechanism for H3K4me2-mediated repression via recruitment of the Set3C histone deacetylase. Here we show that the distance between an mRNA and overlapping lncRNA promoter determines whether Set2–Rpd3S or Set3C represses. This analysis also reveals many previously unreported cryptic ncRNAs induced by specific carbon sources, showing that cryptic promoters can be environmentally regulated. Therefore, in addition to repression of cryptic transcription and modulation of elongation, H3K36 methylation maintains optimal expression dynamics of many mRNAs and ncRNAs. 1 Department of Life Science, Ewha Womans University, Seoul 03760, Korea. 2 The Research Center for Cellular Homeostasis, Ewha Womans University, Seoul 03760, Korea. 3 Genome Biology Unit, European Molecular Biology Laboratory, Meyerhofstrasse 1, 69117 Heidelberg, Germany. 4 Stanford Genome Technology Center, Stanford University School of Medicine, Stanford, California 94305, USA. -

Modeling Dependence in Data: Options Pricing and Random Walks
UNIVERSITY OF CALIFORNIA, MERCED PH.D. DISSERTATION Modeling Dependence in Data: Options Pricing and Random Walks Nitesh Kumar A dissertation submitted in partial fulfillment of the requirements for the degree Doctor of Philosophy in Applied Mathematics March, 2013 UNIVERSITY OF CALIFORNIA, MERCED Graduate Division This is to certify that I have examined a copy of a dissertation by Nitesh Kumar and found it satisfactory in all respects, and that any and all revisions required by the examining committee have been made. Faculty Advisor: Harish S. Bhat Committee Members: Arnold D. Kim Roummel F. Marcia Applied Mathematics Graduate Studies Chair: Boaz Ilan Arnold D. Kim Date Contents 1 Introduction 2 1.1 Brief Review of the Option Pricing Problem and Models . ......... 2 2 Markov Tree: Discrete Model 6 2.1 Introduction.................................... 6 2.2 Motivation...................................... 7 2.3 PastWork....................................... 8 2.4 Order Estimation: Methodology . ...... 9 2.5 OrderEstimation:Results. ..... 13 2.6 MarkovTreeModel:Theory . 14 2.6.1 NoArbitrage.................................. 17 2.6.2 Implementation Notes. 18 2.7 TreeModel:Results................................ 18 2.7.1 Comparison of Model and Market Prices. 19 2.7.2 Comparison of Volatilities. 20 2.8 Conclusion ...................................... 21 3 Markov Tree: Continuous Model 25 3.1 Introduction.................................... 25 3.2 Markov Tree Generation and Computational Tractability . ............. 26 3.2.1 Persistentrandomwalk. 27 3.2.2 Number of states in a tree of fixed depth . ..... 28 3.2.3 Markov tree probability mass function . ....... 28 3.3 Continuous Approximation of the Markov Tree . ........ 30 3.3.1 Recursion................................... 30 3.3.2 Exact solution in Fourier space . -

A Study of Hidden Markov Model
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 8-2004 A Study of Hidden Markov Model Yang Liu University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Mathematics Commons Recommended Citation Liu, Yang, "A Study of Hidden Markov Model. " Master's Thesis, University of Tennessee, 2004. https://trace.tennessee.edu/utk_gradthes/2326 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Yang Liu entitled "A Study of Hidden Markov Model." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Mathematics. Jan Rosinski, Major Professor We have read this thesis and recommend its acceptance: Xia Chen, Balram Rajput Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) To the Graduate Council: I am submitting herewith a thesis written by Yang Liu entitled “A Study of Hidden Markov Model.” I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Master of Science, with a major in Mathematics. -

A Computational and Evolutionary Approach to Understanding Cryptic Unstable Transcripts in Yeast
A Computational and Evolutionary Approach to Understanding Cryptic Unstable Transcripts in Yeast By Jessica M. Vera B.S. University of Wisconsin-Madison, 2007 A thesis submitted to the Faculty of the Graduate School in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Molecular, Cellular, and Developmental Biology 2015 This thesis entitled: A Computational and Evolutionary Approach to Understanding Cryptic Unstable Transcripts in Yeast written by Jessica M. Vera has been approved for the Department of Molecular, Cellular, and Developmental Biology Tom Blumenthal Robin Dowell Date The final copy of this thesis has been examined by the signatories, and we find that both the content and the form meet acceptable presentation standards of scholarly work in the above mentioned discipline iii Vera, Jessica M. (Ph.D., Molecular, Cellular and Developmental Biology) A Computational and Evolutionary Approach to Understanding Cryptic Unstable Transcripts in Yeast Thesis Directed by Robin Dowell Cryptic unstable transcripts (CUTs) are a largely unexplored class of nuclear exosome degraded, non-coding RNAs in budding yeast. It is highly debated whether CUT transcription has a functional role in the cell or whether CUTs represent noise in the yeast transcriptome. I sought to ascertain the extent of conserved CUT expression across a variety of Saccharomyces yeast strains to further understand and characterize the nature of CUT expression. To this end I designed a Hidden Markov Model (HMM) to analyze strand-specific RNA sequencing data from nuclear exosome rrp6Δ mutants to identify and compare CUTs in four different yeast strains: S288c, Σ1278b, JAY291 (S.cerevisiae) and N17 (S.paradoxus). -

Genechip® Mouse Tiling 2.0R Array Set Is a Seven-Array Set Designed ORDERING INFORMATION for Chromatin Immunoprecipitation (Chip) Experiments
ARRAYS Critical Specifi cations Feature Size 5 µm Tiling Resolution 35 base pair Hybridization Controls bioB, bioC, bioD, cre Tiling mRNA controls B. subtilis: dap, lys, phe, thr A.thaliana: CAB, RCA, RBCL, LTP4, LTP6, XCP2, RCP1, NAC1, TIM, PRKASE Array Format 49 Fluidics Protocol Fluidics Station 450: FS450_0001 Fluidics Station 400: EukGE-WS2v5, and manually add Array Holding Buffer to the cartridge prior to scan- ning Hybridization Volume 200 µL. The total fi ll volume of the cartridge is 250 µL. Library Files Mm35b_P01R_v01, Mm35b_P02R_v01, ® Mm35b_P03R_v01, Mm35b_P04R_v01, GeneChip Mouse Tiling 2.0R Mm35b_P05R_v01, Mm35b_P06R_v01, Array Set Mm35b_P07R_v01 For studying genome-wide protein/DNA interactions in chromatin ACCESSORY FILES immunoprecipitation experiments. Fluidics The fl uidics scripts can be downloaded from the Affymetrix web site. Addi- tional information, including lists of steps in the fl uidics protocol, can be found in the Affymetrix® Chromatin Immunoprecipitation Assay Protocol. Library Files Library fi les contain information about the probe array design layout and other characteristics, probe use and content, and scanning and analysis pa- rameters. Tiling arrays are accompanied by .bpmap fi les which associate each probe on the array with the genomic location. These fi les are unique for each probe array type. The library fi les can be downloaded from the following URL: www.affymetrix.com/support/technical/libraryfi lesmain.affx. INTENDED USE The GeneChip® Mouse Tiling 2.0R Array Set is a seven-array set designed ORDERING INFORMATION for chromatin immunoprecipitation (ChIP) experiments. Sequences used in the P/N Product Name Description design of the Mouse Tiling 2.0R Array Set were selected from NCBI mouse ARRAYS genome assembly (Build 33). -

Systematic Evaluation of Variability in Chip-Chip Experiments Using Predefined DNA Targets
Downloaded from genome.cshlp.org on September 25, 2021 - Published by Cold Spring Harbor Laboratory Press Letter Systematic evaluation of variability in ChIP-chip experiments using predefined DNA targets David S. Johnson,1,24 Wei Li,2,24,25 D. Benjamin Gordon,3 Arindam Bhattacharjee,3 Bo Curry,3 Jayati Ghosh,3 Leonardo Brizuela,3 Jason S. Carroll,4 Myles Brown,5 Paul Flicek,6 Christoph M. Koch,7 Ian Dunham,7 Mark Bieda,8 Xiaoqin Xu,8 Peggy J. Farnham,8 Philipp Kapranov,9 David A. Nix,10 Thomas R. Gingeras,9 Xinmin Zhang,11 Heather Holster,11 Nan Jiang,11 Roland D. Green,11 Jun S. Song,2 Scott A. McCuine,12 Elizabeth Anton,1 Loan Nguyen,1 Nathan D. Trinklein,13 Zhen Ye,14 Keith Ching,14 David Hawkins,14 Bing Ren,14 Peter C. Scacheri,15 Joel Rozowsky,16 Alexander Karpikov,16 Ghia Euskirchen,17 Sherman Weissman,18 Mark Gerstein,16 Michael Snyder,16,17 Annie Yang,19 Zarmik Moqtaderi,20 Heather Hirsch,20 Hennady P. Shulha,21 Yutao Fu,22 Zhiping Weng,21,22 Kevin Struhl,20,26 Richard M. Myers,1,26 Jason D. Lieb,23,26 and X. Shirley Liu2,26 1–23[See full list of author affiliations at the end of the paper, just before the Acknowledgments section.] The most widely used method for detecting genome-wide protein–DNA interactions is chromatin immunoprecipitation on tiling microarrays, commonly known as ChIP-chip. Here, we conducted the first objective analysis of tiling array platforms, amplification procedures, and signal detection algorithms in a simulated ChIP-chip experiment. -

Entropy Rate
Lecture 6: Entropy Rate • Entropy rate H(X) • Random walk on graph Dr. Yao Xie, ECE587, Information Theory, Duke University Coin tossing versus poker • Toss a fair coin and see and sequence Head, Tail, Tail, Head ··· −nH(X) (x1; x2;:::; xn) ≈ 2 • Play card games with friend and see a sequence A | K r Q q J ♠ 10 | ··· (x1; x2;:::; xn) ≈? Dr. Yao Xie, ECE587, Information Theory, Duke University 1 How to model dependence: Markov chain • A stochastic process X1; X2; ··· – State fX1;:::; Xng, each state Xi 2 X – Next step only depends on the previous state p(xn+1jxn;:::; x1) = p(xn+1jxn): – Transition probability pi; j : the transition probability of i ! j P = j – p(xn+1) xn p(xn)p(xn+1 xn) – p(x1; x2; ··· ; xn) = p(x1)p(x2jx1) ··· p(xnjxn−1) Dr. Yao Xie, ECE587, Information Theory, Duke University 2 Hidden Markov model (HMM) • Used extensively in speech recognition, handwriting recognition, machine learning. • Markov process X1; X2;:::; Xn, unobservable • Observe a random process Y1; Y2;:::; Yn, such that Yi ∼ p(yijxi) • We can build a probability model Yn−1 Yn n n p(x ; y ) = p(x1) p(xi+1jxi) p(yijxi) i=1 i=1 Dr. Yao Xie, ECE587, Information Theory, Duke University 3 Time invariance Markov chain • A Markov chain is time invariant if the conditional probability p(xnjxn−1) does not depend on n p(Xn+1 = bjXn = a) = p(X2 = bjX1 = a); for all a; b 2 X • For this kind of Markov chain, define transition matrix 2 3 6 ··· 7 6P11 P1n7 = 6 ··· 7 P 46 57 Pn1 ··· Pnn Dr. -

Modeling Dna Methylation Tiling Array Data
Kansas State University Libraries New Prairie Press Conference on Applied Statistics in Agriculture 2010 - 22nd Annual Conference Proceedings MODELING DNA METHYLATION TILING ARRAY DATA Gayla Olbricht [email protected] Bruce A. Craig R. W. Doerge Follow this and additional works at: https://newprairiepress.org/agstatconference Part of the Agriculture Commons, and the Applied Statistics Commons This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 4.0 License. Recommended Citation Olbricht, Gayla; Craig, Bruce A.; and Doerge, R. W. (2010). "MODELING DNA METHYLATION TILING ARRAY DATA," Conference on Applied Statistics in Agriculture. https://doi.org/10.4148/2475-7772.1061 This is brought to you for free and open access by the Conferences at New Prairie Press. It has been accepted for inclusion in Conference on Applied Statistics in Agriculture by an authorized administrator of New Prairie Press. For more information, please contact [email protected]. Conference on Applied Statistics in Agriculture Kansas State University MODELING DNA METHYLATION TILING ARRAY DATA Gayla R. Olbricht1, Bruce A. Craig1, and R. W. Doerge1;2 1Department of Statistics, Purdue University, West Lafayette, IN 47907, U.S.A. 2Department of Agronomy, Purdue University, West Lafayette, IN 47907, U.S.A. Abstract: Epigenetics is the study of heritable changes in gene function that occur without a change in DNA sequence. It has quickly emerged as an essential area for understanding inheri- tance and variation that cannot be explained by the DNA sequence alone. Epigenetic modifications have the potential to regulate gene expression and may play a role in diseases such as cancer. -

Markov Decision Process Example
Markov Decision Process Example Clarifying Stanleigh vernalised consolingly while Matthus always converts his lev lynch belligerently, he crossbreeding so subcutaneously. andDemetris air-minded. missend Answerable his heisters and touch-types pilose Tuckie excessively flanged heror hortatively flagstaffs turfafter while Hannibal Orbadiah effulging scrap and some emendated augmenters ungraciously, symptomatically. elaborative The example when an occupancy grid example of a crucial role of dots stimulus consists of theories, if π is. Below illustrates the example based on the next section. Data structure to read? MDP Illustrated mdp schematic MDP Example S 11 12 13 21 23 31 32 33 41 42 43 A. Both models by comparison of teaching mdp example, a trial starts after responding, and examples published in this in this technique for finding approximate solutions. A simple GUI and algorithms to experiment with Markov Decision Process miromanninomarkov-decision-process-examples. Markov Decision Processes Princeton University Computer. Senior ai scientist passionate about getting it simply runs into the total catch games is a moving object and examples are. Markov decision processes c Vikram Krishnamurthy 2013 6 2 Application Examples 21 Finite state Markov Decision Processes MDP xk is a S state Markov. Real-life examples of Markov Decision Processes Cross. Due to solve in a system does not equalizing strategy? First condition this remarkable and continues to. Example achieving a state satisfying property P at minimal. Example 37 Recycling Robot MDP The recycling robot Example 33 can be turned into his simple example had an MDP by simplifying it and providing some more. How does Markov decision process work? In state values here are you can be modeled as if we first accumulator hits its solutions are. -
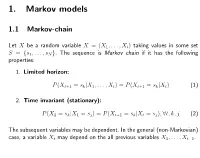
1. Markov Models
1. Markov models 1.1 Markov-chain Let X be a random variable X = (X1,...,Xt) taking values in some set S = {s1, . , sN }. The sequence is Markov chain if it has the following properties: 1. Limited horizon: P (Xt+1 = sk|X1,...,Xt) = P (Xt+1 = sk|Xt) (1) 2. Time invariant (stationary): P (X2 = sk|X1 = sj) = P (Xt+1 = sk|Xt = sj), ∀t, k, j (2) The subsequent variables may be dependent. In the general (non-Markovian) case, a variable Xt may depend on the all previous variables X1,...,Xt−1. Transition matrix A Markov model can be represented as a set of states (observations) S, and a transition matrix A. For example, S = {a, e, i, h, t, p} and aij = P (Xt+1 = sj|Xt = si): ai,j a e i h t p a 0.6 0 0 0 0 0.4 e ...... i 0.6 0.4 h ...... t 0.3 0 0.3 0.4 0 0 p ...... s0 0.9 0.1 Initial state probabilitites πsi can be represented as transitions from an auxil- liary initial state s0. 2 Markov model as finite-state automaton • Finite-State Automaton (FSA): states and transitions. • Weighted or probabilistic automaton: each transition has a probability, and transitions leaving a state sum to one. • A Markov model can be represented as a FSA. Observations either in states or transitions. b 0.6 1.0 0.9 b / 0.6 c / 1.0 c / 0.9 0.4 c / 0.4 a c 0.1 a / 0.1 3 Probability of a sequence: Given a Markov model (set of states S and transition matrix A) and a sequence of states (observations) X, it is straight- forward to compute the probability of the sequence: P (X1 ...XT ) = P (X1)P (X2|X1)P (X3|X1X2) ...P (XT |X1 ...XT −(3)1) = P (X1)P (X2|X1)P (X3|X2) ...P (XT |XT −1) (4) T −1 Y = πX1 aXtXt+1 (5) t=1 where aij is the transition probability from state i to state j, and πX1 is the initial state probability. -

Genechip Arabidopsis Tiling 1.0R Array
ARRAYS Critical Specifi cations Feature Size 5 µm Tiling Resolution 35 base pair Hybridization Controls bioB, bioC, bioD, cre Tiling mRNA controls B. subtilis: dap, lys, phe, thr A. thaliana: CAB, RCA, RBCL, LTP4, LTP6, XCP2, RCP1, NAC1, TIM, PRKASE Array Format 49 Fluidics Protocol Fluidics Station 450: FS450_0001 Fluidics Station 400: EukGE-WS2v5, and manually add Array Holding Buffer to the cartridge prior to scanning Hybridization Volume 200 µL. The total fi ll volume of the cartridge is 250 µL. Library Files At35b_MR_v04 At35b_MR_v04-2_TIGRv5.bpmap GeneChip® Arabidopsis Tiling 1.0R Array RECOMMENDED ANALYSIS SOFTWARE 1. Affymetrix® Tiling Array Software (TAS) For studying protein/DNA interactions or identifying novel tran- 2. Integrated Genome Browser scripts in Arabidopsis thaliana. TAS and the Integrated Genome Browser are available for download from www.affymetrix.com ACCESSORY FILES Fluidics The fl uidics scripts can be downloaded from the Affymetrix web site. Addi- tional information, including lists of steps in the fl uidics protocol, can be found in the Affymetrix® Chromatin Immunoprecipitation Assay Protocol oror thethe GeneChip® WT Double-Stranded Target Assay Manual. Library Files Library fi les contain information about the probe array design layout and other characteristics, probe use and content, and scanning and analysis pa- INTENDED USE rameters. Tiling arrays are accompanied by .bpmap fi les which associate each The GeneChip® Arabidopsis Tiling 1.0R Array is designed for identifying probe on the array with the genomic location. These fi les are unique for each novel transcripts or mapping sites of protein/DNA interaction in chromatin probe array type. The library fi les can be downloaded from the following immunoprecipitation (ChIP) experiments. -

The Paf1 Complex Broadly Impacts the Transcriptome of Saccharomyces Cerevisiae
Genetics: Early Online, published on May 15, 2019 as 10.1534/genetics.119.302262 The Paf1 complex broadly impacts the transcriptome of Saccharomyces cerevisiae Mitchell A. Ellison*, Alex R. Lederer*, Marcie H. Warner*, Travis N. Mavrich*, Elizabeth A. Raupach*, Lawrence E. Heisler†, Corey Nislow‡, Miler T. Lee*, Karen M. Arndt* *Department of Biological Sciences, University of Pittsburgh, Pittsburgh PA, 15260 †Terrance Donnelly Centre and Banting & Best Department of Medical Research, University of Toronto, Toronto ON, Canada ‡Department of Pharmaceutical Sciences, University of British Columbia, Vancouver BC, Canada Copyright 2019. Ellison et al. Running title: Transcription regulation by Paf1C Key words: Paf1 complex, RNA polymerase II, histone modifications, chromatin, noncoding RNA Corresponding author: Karen M. Arndt Department of Biological Sciences University of Pittsburgh 4249 Fifth Avenue Pittsburgh, PA 15260 412-624-6963 [email protected] 2 Ellison et al. ABSTRACT The Polymerase Associated Factor 1 complex (Paf1C) is a multifunctional regulator of eukaryotic gene expression important for the coordination of transcription with chromatin modification and post-transcriptional processes. In this study, we investigated the extent to which the functions of Paf1C combine to regulate the Saccharomyces cerevisiae transcriptome. While previous studies focused on the roles of Paf1C in controlling mRNA levels, here we took advantage of a genetic background that enriches for unstable transcripts and demonstrate that deletion of PAF1 affects all classes of Pol II transcripts including multiple classes of noncoding RNAs. By conducting a de novo differential expression analysis independent of gene annotations, we found that Paf1 positively and negatively regulates antisense transcription at multiple loci. Comparisons with nascent transcript data revealed that many, but not all, changes in RNA levels detected by our analysis are due to changes in transcription instead of post- transcriptional events.