Breeding Laboratories (Hino, Japan)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Ultraviolet-Visible Reference Spectra C
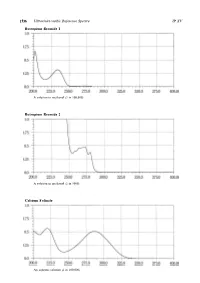
1536 Ultraviolet-visible Reference Spectra JP XV Butropium Bromide 1 A solution in methanol (1 in 100,000) Butropium Bromide 2 A solution in methanol (1 in 5000) Calcium Folinate An aqueous solution (1 in 100,000) JP XV Ultraviolet-visible Reference Spectra 1537 Camostat Mesilate An aqueous solution (1 in 100,000) Carbamazepine The sample solution obtained in the Assay Carbazochrome Sodium Sulfonate Hydrate An aqueous solution (1 in 100,000) 1538 Ultraviolet-visible Reference Spectra JP XV Carbidopa Hydrate A solution prepared as follows: Dissolve 0.01 g in 250 mL of a solution of hydrochloric acid in methanol (9 in 1000) Carmofur A solution in a mixture of methanol and phosphoric acid-acetic acid-boric acid buffer solution, pH 2.0 (9:1) (1 in 100,000) Carteolol Hydrochloride An aqueous solution (1 in 100,000) JP XV Ultraviolet-visible Reference Spectra 1539 Carumonam Sodium An aqueous solution (3 in 100,000) Cefaclor An aqueous solution (1 in 50,000) Cefadroxil An aqueous solution (1 in 50,000) 1540 Ultraviolet-visible Reference Spectra JP XV Cefalexin An aqueous solution (3 in 100,000) Cefalotin Sodium An aqueous solution (1 in 50,000) Cefapirin Sodium An aqueous solution (3 in 200,000) JP XV Ultraviolet-visible Reference Spectra 1541 Cefatrizine Propylene Glycolate An aqueous solution (1 in 50,000) Cefazolin Sodium An aqueous solution (1 in 50,000) Cefbuperazone Sodium An aqueous solution (1 in 50,000) 1542 Ultraviolet-visible Reference Spectra JP XV Cefditoren Pivoxil A solution in methanol (1 in 50,000) Cefixime A solution in 0.1 -

00246-2019.Supptables
1 Table S1. List of ICD-10 codes identifying comorbidities during hospitalization for COPD exacerbation COPD J41, J42, J43, J440, J441, J449 COPD exacerbation J10, J11, J13, J14, J15, J16, J18, J20, J21, J22, J46, J170, J171, J178, J440, J441, J851, J12, A481, B012, B052, B250 Bacterial pneumonia J100, J110, J12, J13, J14, J15, J16, J170, J178, J18, J851, A481 Lung cancer C34 Other malignancy C00-26, C30-33, C37-41, C43-58, C60-76 Diabetes/abnormal glucose tolerance E10-14, R703 Bone fracture/osteoporosis M80-84 Interstitial pneumonia B221, J701, J704, J841, J848, J849, J990, J991, M321, M330, M331, M332, M351 Bronchial asthma J45, J46 Bronchiectasis J40, J41, J42, J47 Pulmonary thromboembolism I26 Mycobacterium infection A150-154, A156-159, A161-162, A165, A168-169, A19, A310, A319 Mycotic infection A420, A43, B37, B380-382, B390-392, B400-402, B410, B420, B440, B441, B449, B460, J172 Cor pulmonare I27 Congestive heart failure E059, I46, I50, I099, I110 Ischemic heart disease I20-25 Tachycardia I47-49, R000, T818 Autoimmune disease M05, M06, M08, M30-35 Stroke I60-64 Liver dysfunction B89, B181, B182, B659, B661, K702, K703, K72, K74, K761, K762, K763, K766, K767 Renal failure E102, E112, E142, I120, N17-19 GERD K21 Constipation or ileus K56, K590 Prostate hypertrophy N40 Abbreviations: COPD, chronic obstructive pulmonary disease; GERD, gastro-esophageal reflux disease 2 Table S2. List of baseline characteristics, comorbidities, and treatments before and during hospitalization for COPD exacerbation Baseline characteristics Sex, fiscal -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Consideration of Antibacterial Medicines As Part Of
Consideration of antibacterial medicines as part of the revisions to 2019 WHO Model List of Essential Medicines for adults (EML) and Model List of Essential Medicines for children (EMLc) Section 6.2 Antibacterials including Access, Watch and Reserve Lists of antibiotics This summary has been prepared by the Health Technologies and Pharmaceuticals (HTP) programme at the WHO Regional Office for Europe. It is intended to communicate changes to the 2019 WHO Model List of Essential Medicines for adults (EML) and Model List of Essential Medicines for children (EMLc) to national counterparts involved in the evidence-based selection of medicines for inclusion in national essential medicines lists (NEMLs), lists of medicines for inclusion in reimbursement programs, and medicine formularies for use in primary, secondary and tertiary care. This document does not replace the full report of the WHO Expert Committee on Selection and Use of Essential Medicines (see The selection and use of essential medicines: report of the WHO Expert Committee on Selection and Use of Essential Medicines, 2019 (including the 21st WHO Model List of Essential Medicines and the 7th WHO Model List of Essential Medicines for Children). Geneva: World Health Organization; 2019 (WHO Technical Report Series, No. 1021). Licence: CC BY-NC-SA 3.0 IGO: https://apps.who.int/iris/bitstream/handle/10665/330668/9789241210300-eng.pdf?ua=1) and Corrigenda (March 2020) – TRS1021 (https://www.who.int/medicines/publications/essentialmedicines/TRS1021_corrigenda_March2020. pdf?ua=1). Executive summary of the report: https://apps.who.int/iris/bitstream/handle/10665/325773/WHO- MVP-EMP-IAU-2019.05-eng.pdf?ua=1. -

WO 2010/025328 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 4 March 2010 (04.03.2010) WO 2010/025328 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/00 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (21) International Application Number: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, PCT/US2009/055306 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 28 August 2009 (28.08.2009) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (26) Publication Language: English SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/092,497 28 August 2008 (28.08.2008) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): FOR¬ GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, EST LABORATORIES HOLDINGS LIMITED [IE/ ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, —]; 18 Parliament Street, Milner House, Hamilton, TM), European (AT, BE, BG, CH, CY, CZ, DE, DK, EE, Bermuda HM12 (BM). -

Computational Antibiotics Book
Andrew V DeLong, Jared C Harris, Brittany S Larcart, Chandler B Massey, Chelsie D Northcutt, Somuayiro N Nwokike, Oscar A Otieno, Harsh M Patel, Mehulkumar P Patel, Pratik Pravin Patel, Eugene I Rowell, Brandon M Rush, Marc-Edwin G Saint-Louis, Amy M Vardeman, Felicia N Woods, Giso Abadi, Thomas J. Manning Computational Antibiotics Valdosta State University is located in South Georgia. Computational Antibiotics Index • Computational Details and Website Access (p. 8) • Acknowledgements (p. 9) • Dedications (p. 11) • Antibiotic Historical Introduction (p. 13) Introduction to Antibiotic groups • Penicillin’s (p. 21) • Carbapenems (p. 22) • Oxazolidines (p. 23) • Rifamycin (p. 24) • Lincosamides (p. 25) • Quinolones (p. 26) • Polypeptides antibiotics (p. 27) • Glycopeptide Antibiotics (p. 28) • Sulfonamides (p. 29) • Lipoglycopeptides (p. 30) • First Generation Cephalosporins (p. 31) • Cephalosporin Third Generation (p. 32) • Fourth-Generation Cephalosporins (p. 33) • Fifth Generation Cephalosporin’s (p. 34) • Tetracycline antibiotics (p. 35) Computational Antibiotics Antibiotics Covered (in alphabetical order) Amikacin (p. 36) Cefempidone (p. 98) Ceftizoxime (p. 159) Amoxicillin (p. 38) Cefepime (p. 100) Ceftobiprole (p. 161) Ampicillin (p. 40) Cefetamet (p. 102) Ceftoxide (p. 163) Arsphenamine (p. 42) Cefetrizole (p. 104) Ceftriaxone (p. 165) Azithromycin (p.44) Cefivitril (p. 106) Cefuracetime (p. 167) Aziocillin (p. 46) Cefixime (p. 108) Cefuroxime (p. 169) Aztreonam (p.48) Cefmatilen ( p. 110) Cefuzonam (p. 171) Bacampicillin (p. 50) Cefmetazole (p. 112) Cefalexin (p. 173) Bacitracin (p. 52) Cefodizime (p. 114) Chloramphenicol (p.175) Balofloxacin (p. 54) Cefonicid (p. 116) Cilastatin (p. 177) Carbenicillin (p. 56) Cefoperazone (p. 118) Ciprofloxacin (p. 179) Cefacetrile (p. 58) Cefoselis (p. 120) Clarithromycin (p. 181) Cefaclor (p. -

Pharmaceutical Appendix to the Harmonized Tariff Schedule
Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Front Matter
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY VOLUME 27 * JUNE 1985 * NUMBER 6 Leon H. Schmidt, Edlitor in Chief (1985) Robert C. Moellering, Jr., Etlitor (1987) Unlivevr.sity of Alabana in Bi-inif'/igllam NVw' Etiglant Dco(t0iesS ILHoJspital Birmiiinghattn Bostoti, Ma.so(lahsetts Herbert L. Ennis, Editor (1987) Christine C. Sanders, Editor, (1989) Roche Institiute of AMole(ula(l Biology Creightoni Universitv Sc(0hoolfoV'(lidcil( Nmutlev, Newi, Jer-seyv Omaha, Nebraska Robert L. Hamill, Editor (1985) Peter G. Welling, Eklitor (1988) Eli Lil//, & Colfnpany l(c. Warl Clr-La/Ub/t Co. Indiaina(lpolis, lntdianlal A tin Arbor, Michigall George A. Jacoby, Jr., Elitor (1985) John A. Washington II, Editor (1986) Massachusetts Generatil Hospital Mayo Clinic Boston Rochester. NIilhlC 'vota EDITORIAL BOARD Norris Allen (1986) Arthur English (1986) Joan Lusk (1986) Nlerle Sande (198>() Vincent T. Andriole (1987) Robert J. Fass (1985) R. Luthy (1986) NV. Eugene Sanders, Jr. (1987) Bascom F. Anthony (1985) Stuart Feldman (1985) Francis L. Macrina (1985) WV. NMichael Scheld (1986) George R. Aronoff (1986) Sydney Finegold (1985) Gerald L. Mandell (1986) Jerome J. Schentag (1985) Robert Austrian (1986) Robert J. Fitzgerald (1986) Garv R. Matzke (1986) Raymond F. Schinazi (1986) Richard H. Baltz (1987) Martin Forbes (1986) George H. McCracken (1987) Fritz D. Schoenknecht (1986) Rashmi H. Barbhaiya (1986) John N. Galgiani (1987) Antone A. Medeiros (1987) F. C. Sciavolino (1985) Arthur L. Barry (1986) Dale N. Gerding (1985) Gerald Medoff (1986) VW'illiam NI. Shannon (1986) John G. Bartlett (1987) Anthony J. Glazko (1987) NMichael Miller (1987) Charles Shipman, Jr. (1985) Michael Barza (1985) Irving H. -

Pharmaceutical Appendix to the Harmonized Tariff Schedule
Harmonized Tariff Schedule of the United States (2020) Revision 8 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 8 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Pharmaceutical Appendix to the Harmonized Tariff
Harmonized Tariff Schedule of the United States (2019) Revision 11 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2019) Revision 11 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Directed Molecular Evolution of Fourth-Generation Cephalosporin Resistance in Wellington Moore Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2011 Directed molecular evolution of fourth-generation cephalosporin resistance in Wellington Moore Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Medical Sciences Commons Recommended Citation Moore, Wellington, "Directed molecular evolution of fourth-generation cephalosporin resistance in" (2011). Graduate Theses and Dissertations. 10107. https://lib.dr.iastate.edu/etd/10107 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Directed molecular evolution of fourth-generation cephalosporin resistance in Salmonella and Yersinia by Wellington Moore A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Biomedical Science (Pharmacology) Program of Study Committee: Steve Carlson, Major Professor Timothy Day Ronald Griffith Iowa State University Ames, Iowa 2011 ii TABLE OF CONTENTS LIST OF FIGURES………………………………………………………………………iii LIST OF TABLES………………………………………………………………………..iv ABSTRACT……………………………………………………………………………….v CHAPTER 1. INTRODUCTION…………………………………………………………1 Review of B-Lactam antimicrobials………………………………...………1