Anatomical Study of Myofascial Continuity in the Anterior Region of the Upper Limb
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Study on the Absence of Palmaris Longus in a Multi-Racial Population
108472 NV-OA7 pg26-28.qxd 11/05/2007 05:02 PM Page 26 (Black plate) Malaysian Orthopaedic Journal 2007 Vol 1 No 1 SA Roohi, etal A Study on the Absence of Palmaris Longus in a Multi- racial Population SA Roohi, MS (Ortho) (UKM), L Choon-Sian, MD (UKM), A Shalimar, MS (Ortho) (UKM), GH Tan, MS (Ortho) (UKM), AS Naicker, M Med Rehab (UM) Hospital Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia ABSTRACT Most standard textbooks of hand surgery quote the prevalence of absence of palmaris longus at around 15%3-5. Palmaris longus is a dispensable muscle with a long tendon However, this figure varies considerably in different ethnic which is very useful in reconstructive surgery. It is absent groups. A study by Thompson et al6 on 300 Caucasian 2.8 to 24% of the population depending on the race/ethnicity subjects found that palmaris longus was absent unilaterally in studied. Four hundred and fifty healthy subjects (equally 16%, and bilaterally in 9% of the study sample for an overall distributed among Malaysia’s 3 major ethnic groups) were prevalence of absence of 24%. Similarly, George7 noted on clinically examined for the presence or absence of palmaris 276 cadavers of European descent that its absence was 13% longus. This tendon was found to be absent unilaterally in unilaterally, 8.7% bilaterally for an overall absence of 15.2%. 6.4% of study subjects, and bilaterally in 2.9% of study Another cadaveric study by Vanderhooft8 in Seattle, USA participants. Malays have a high prevalence of palmaris reported its overall absence to be 12%. -

Ultrasonograpic Assessment of Relationship Between the Palmaris Longus Tendon and the Flexor Retinacular Ligament and the Palmar Aponeurosis of the Hand
Original Article Ultrasonograpic Assessment of Relationship Between the Palmaris Longus Tendon and the Flexor Retinacular Ligament and the Palmar Aponeurosis of the Hand Kadir Ertem1, Ahmet Sığırcı2, Salih Karaca1, Aykut Sığırcı3, Yunus Karakoç4, Saim Yoloğlu5 İnonu University, Faculty of Medicine, ABSTRACT Departments of Orthopedics and Trauma- tology1, Radioloy2, Physiology4 and Biosta- Aim: This study aimed to evaluate the presence of the Palmaris Longus tistics5, Malatya, Turkey Tendon (PLT) and the relationship between the Flexor Retinacular Ligament (FRL) and the Palmar Aponeurosis (PA) of the hand. 319 Mayıs University, Faculty of Medicine, Departments of Orthopaedics and Trauma- Method: 62 voluntary subjects (31 female, 31 male students and per- tology, Samsun, Turkey sonnel from the Inonu University, at the average age 28.38 ± 6.86 years ranging from 19 to 48 years) took part in this study using ultrasound. Eur J Gen Med 2010;7(2):161-166 Received: 16.05.2009 Result: Significant differences were found in the PA p-m-d diameters of subjects between with and without PLT bilaterally, on the right Accepted: 06.07.2009 and the left hand (p<0.05), whereas there was no meaningful differ- ence considering FRL diameters (p>0.05). Furthermore, this ultraso- nographic assessment revealed the continuity of collagen bunches of the PL tendon up to FRL, but not PA. Conclusion: Although not demonstrated by ultrasonography here, the increased thickness of the PA in subjects with a PLT supports the find- ings in the literature in which the structural -
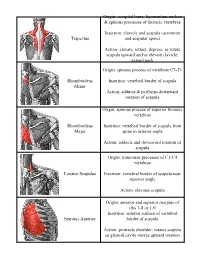
Trapezius Origin: Occipital Bone, Ligamentum Nuchae & Spinous Processes of Thoracic Vertebrae Insertion: Clavicle and Scapul
Origin: occipital bone, ligamentum nuchae & spinous processes of thoracic vertebrae Insertion: clavicle and scapula (acromion Trapezius and scapular spine) Action: elevate, retract, depress, or rotate scapula upward and/or elevate clavicle; extend neck Origin: spinous process of vertebrae C7-T1 Rhomboideus Insertion: vertebral border of scapula Minor Action: adducts & performs downward rotation of scapula Origin: spinous process of superior thoracic vertebrae Rhomboideus Insertion: vertebral border of scapula from Major spine to inferior angle Action: adducts and downward rotation of scapula Origin: transverse precesses of C1-C4 vertebrae Levator Scapulae Insertion: vertebral border of scapula near superior angle Action: elevates scapula Origin: anterior and superior margins of ribs 1-8 or 1-9 Insertion: anterior surface of vertebral Serratus Anterior border of scapula Action: protracts shoulder: rotates scapula so glenoid cavity moves upward rotation Origin: anterior surfaces and superior margins of ribs 3-5 Insertion: coracoid process of scapula Pectoralis Minor Action: depresses & protracts shoulder, rotates scapula (glenoid cavity rotates downward), elevates ribs Origin: supraspinous fossa of scapula Supraspinatus Insertion: greater tuberacle of humerus Action: abduction at the shoulder Origin: infraspinous fossa of scapula Infraspinatus Insertion: greater tubercle of humerus Action: lateral rotation at shoulder Origin: clavicle and scapula (acromion and adjacent scapular spine) Insertion: deltoid tuberosity of humerus Deltoid Action: -

Anatomy and Physiology II
Anatomy and Physiology II Review Bones of the Upper Extremities Muscles of the Upper Extremities Anatomy and Physiology II Review Bones of the Upper Extremities Questions From Shoulder Girdle Lecture • Can you name the following structures? A – F • Acromion F – B B • Spine of the Scapula G – C • Medial (Vertebral) Border H – E C • Lateral (Axillary) Border – A • Superior Angle E I – D • Inferior Angle – G • Head of the Humerus D – H • Greater Tubercle of Humerus – I • Deltoid Tuberosity Questions From Shoulder Girdle Lecture • Would you be able to find the many of the same landmarks on this view (angles, borders, etc)? A • Can you name the following? – D • Coracoid process of scapula C – C D B • Lesser Tubercle – A • Greater Tubercle – B • Bicipital Groove (Intertubercular groove) Questions From Upper Extremities Lecture • Can you name the following structures? – B • Lateral epicondyle – A • Medial epicondyle A B Questions From Upper Extremities Lecture • Can you name the following landmarks? – C • Olecranon process – A • Head of the radius – B D • Medial epicondyle B A – D C • Lateral epicondyle Questions From Upper Extremities Lecture • Can you name the following bones and landmarks? – Which bone is A pointing to? • Ulna – Which bone is B pointing A to? • Radius E – C B • Styloid process of the ulna – D • Styloid process of the radius C – E D • Interosseous membrane of forearm Questions From Upper Extremities Lecture • Can you name the following bony landmarks? – Which landmark is A pointing to? • Lateral epicondyle of humerus – Which -

Anatomical Considerations of Fascial Release in Ulnar Nerve Transposition: a Concept Revisited
LABORATORY INVESTIGATION J Neurosurg 123:1216–1222, 2015 Anatomical considerations of fascial release in ulnar nerve transposition: a concept revisited Mark A. Mahan, MD,1 Jaime Gasco, MD,2 David B. Mokhtee, MD,3 and Justin M. Brown, MD4 1Division of Neurological Surgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona; 2Division of Neurological Surgery, University of Texas Medical Branch, Galveston, Texas; 3Tulsa Bone and Joint Associates, Tulsa, Oklahoma; and 4Division of Neurosurgery, University of California, San Diego, La Jolla, California OBJECT Surgical transposition of the ulnar nerve to alleviate entrapment may cause otherwise normal structures to become new sources of nerve compression. Recurrent or persistent neuropathy after anterior transposition is commonly attributable to a new distal compression. The authors sought to clarify the anatomical relationship of the ulnar nerve to the common aponeurosis of the humeral head of the flexor carpi ulnaris (FCU) and flexor digitorum superficialis (FDS) muscles following anterior transposition of the nerve. METHODS The intermuscular septa of the proximal forearm were explored in 26 fresh cadaveric specimens. The fibrous septa and common aponeurotic insertions of the flexor-pronator muscle mass were evaluated in relation to the ulnar nerve, with particular attention to the effect of transposition upon the nerve in this region. RESULTS An intermuscular aponeurosis associated with the FCU and FDS muscles was present in all specimens. Transposition consistently resulted in angulation of the nerve during elbow flexion when this fascial septum was not released. The proximal site at which the nerve began to traverse this fascial structure was found to be an average of 3.9 cm (SD 0.7 cm) from the medial epicondyle. -

Section 1 Upper Limb Anatomy 1) with Regard to the Pectoral Girdle
Section 1 Upper Limb Anatomy 1) With regard to the pectoral girdle: a) contains three joints, the sternoclavicular, the acromioclavicular and the glenohumeral b) serratus anterior, the rhomboids and subclavius attach the scapula to the axial skeleton c) pectoralis major and deltoid are the only muscular attachments between the clavicle and the upper limb d) teres major provides attachment between the axial skeleton and the girdle 2) Choose the odd muscle out as regards insertion/origin: a) supraspinatus b) subscapularis c) biceps d) teres minor e) deltoid 3) Which muscle does not insert in or next to the intertubecular groove of the upper humerus? a) pectoralis major b) pectoralis minor c) latissimus dorsi d) teres major 4) Identify the incorrect pairing for testing muscles: a) latissimus dorsi – abduct to 60° and adduct against resistance b) trapezius – shrug shoulders against resistance c) rhomboids – place hands on hips and draw elbows back and scapulae together d) serratus anterior – push with arms outstretched against a wall 5) Identify the incorrect innervation: a) subclavius – own nerve from the brachial plexus b) serratus anterior – long thoracic nerve c) clavicular head of pectoralis major – medial pectoral nerve d) latissimus dorsi – dorsal scapular nerve e) trapezius – accessory nerve 6) Which muscle does not extend from the posterior surface of the scapula to the greater tubercle of the humerus? a) teres major b) infraspinatus c) supraspinatus d) teres minor 7) With regard to action, which muscle is the odd one out? a) teres -

Powerpoint Handout: Lab 10, Arm, Cubital Fossa, and Elbow Joint
PowerPoint Handout: Lab 10, Arm, Cubital Fossa, and Elbow Joint Slide Title Slide Number Slide Title Slide Number Osteology of Elbow Complex Slide 2 Supracondylar Fractures Slide 16 Review of Superficial Veins in Arm Slide 3 Radial Head Fracture Slide 17 Arm: Introduction Slide 4 Median Nerve Lesion at Elbow Slide 18 Arm: Anterior Compartment Muscles Slide 5 Radial Nerve Slide 19 Arm: Posterior Compartment Muscles Slide 6 Humeral Shaft Fracture Slide 20 Cubital Fossa Slide 7 Medial Cutaneous Nerve of Arm Slide 21 Brachial Artery Slide 8 Elbow Joint Complex Slide 22 Brachial Artery Pulse Slide 9 Elbow Capsule & Ligaments Slide 23 Bicipital Aponeurosis Slide 10 Nursemaid’s Elbow Slide 24 Musculocutaneous Nerve Slide 11 Olecranon Bursitis (Student’s Bursitis) Slide 25 Ulnar Nerve Slide 12 Ulnar Nerve Lesion at Elbow Slide 13 Ulnar Nerve Lesion at Wrist Slide 14 Median Nerve Slide 15 Osteology of Elbow Complex To adequately review the learning objectives covering osteology of the distal humerus, radius, and ulna, view the Lower Limb Osteology and Medical Imaging Guide. Review of Superficial Veins in Arm The cephalic and basilic veins are the main superficial veins of the upper limb. They originate from the dorsal venous network on the dorsum of the hand. • The cephalic vein ascends along the anterolateral aspect of the forearm and arm. It then follows the superior border of the pectoralis major muscle to enter the deltopectoral triangle. It ultimately joins the axillary vein after passing through the clavipectoral fascia. • The basilic vein ascends along the medial forearm and the arm. In the arm, it passes deep to the brachial fascia where it courses in close proximity to the brachial artery and medial cutaneous nerve of the forearm along its path into the axilla. -

Supplemental Methods
Supplemental Methods: Patients: Inclusion criteria were patients 18 years of age and older with a lower trunk or C8-T1 pattern of brachial plexus injury and a minimum of 6 months postoperative follow-up. The indication for brachialis to FDP muscle transfer was a lack of active finger flexion in patients with a history of lower trunk or C8-T1 pattern of brachial plexus injury at greater than 9 months from injury with no sign of nerve recovery on clinical exam as well as electromyogram. Additionally, triceps motor function of BRMC grade 2+ or greater was required to provide significant antagonist force to balance the elbow flexion force generated with firing of the brachialis muscle transfer. Patients were excluded if they were younger than 18 years of age, had a triceps of grade 2 or less, or sustained other injuries which precluded the use of the brachialis or FDP for tendon transfers. Patient demographic data including age, sex, BMI, smoking status, age at time of injury, mechanism of injury, co-morbidities, and history of previous reconstructive surgery. Patients were follow-up at regular intervals following surgery for functional assessment until their functional level had plateaued. Patients initially received a full evaluation for motor strength by the three senior authors (AYS, ATB, RJS), including but not limited to trapezius, supraspinatus, infraspinatus, deltoid, biceps, triceps, pronator teres, flexor digitorum profundus, extensor digitorum communis, extensor carpi radialis longus and brevis, and first dorsal interosseous motor strength. Motor strength was graded using a modified British Medical Research Council Grade (BMRC) 2,15. BMRC grading is reported as follows: M0 – no contraction, no joint motion, and no EMG reinnervation; M1 – EMG reinnervation, but no joint motion; M2 – perceptible joint motion, however insufficient power to act against gravity; M3 – muscle act against gravity; M4 – muscle acts against resistance; and M5 – muscle acts against strong resistance. -
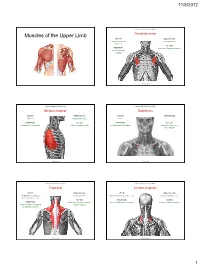
Muscles of the Upper Limb.Pdf
11/8/2012 Muscles Stabilizing Pectoral Girdle Muscles of the Upper Limb Pectoralis minor ORIGIN: INNERVATION: anterior surface of pectoral nerves ribs 3 – 5 ACTION: INSERTION: protracts / depresses scapula coracoid process (scapula) (Anterior view) Muscles Stabilizing Pectoral Girdle Muscles Stabilizing Pectoral Girdle Serratus anterior Subclavius ORIGIN: INNERVATION: ORIGIN: INNERVATION: ribs 1 - 8 long thoracic nerve rib 1 ---------------- INSERTION: ACTION: INSERTION: ACTION: medial border of scapula rotates scapula laterally inferior surface of scapula stabilizes / depresses pectoral girdle (Lateral view) (anterior view) Muscles Stabilizing Pectoral Girdle Muscles Stabilizing Pectoral Girdle Trapezius Levator scapulae ORIGIN: INNERVATION: ORIGIN: INNERVATION: occipital bone / spinous accessory nerve transverse processes of C1 – C4 dorsal scapular nerve processes of C7 – T12 ACTION: INSERTION: ACTION: INSERTION: stabilizes / elevates / retracts / upper medial border of scapula elevates / adducts scapula acromion / spine of scapula; rotates scapula lateral third of clavicle (Posterior view) (Posterior view) 1 11/8/2012 Muscles Stabilizing Pectoral Girdle Muscles Moving Arm Rhomboids Pectoralis major (major / minor) ORIGIN: INNERVATION: ORIGIN: INNERVATION: spinous processes of C7 – T5 dorsal scapular nerve sternum / clavicle / ribs 1 – 6 dorsal scapular nerve INSERTION: ACTION: INSERTION: ACTION: medial border of scapula adducts / rotates scapula intertubucular sulcus / greater tubercle flexes / medially rotates / (humerus) adducts -

The Impact of Palmaris Longus Muscle on Function in Sports: an Explorative Study in Elite Tennis Players and Recreational Athletes
Journal of Functional Morphology and Kinesiology Article The Impact of Palmaris Longus Muscle on Function in Sports: An Explorative Study in Elite Tennis Players and Recreational Athletes Julie Vercruyssen 1,*, Aldo Scafoglieri 2 and Erik Cattrysse 2 1 Faculty of Physical Education and Physiotherapy, Master of Science in Manual therapy, Vrije Universiteit Brussel, Laarbeeklaan 103, 1090 Brussels, Belgium 2 Faculty of Physical Education and Physiotherapy, Department of Experimental Anatomy, Vrije Universiteit Brussel, Laarbeeklaan 103, 1090 Brussels, Belgium; [email protected] (A.S.); [email protected] (E.C.) * Correspondence: [email protected]; Tel.: +32-472-741-808 Academic Editor: Giuseppe Musumeci Received: 21 February 2016; Accepted: 24 March 2016; Published: 13 April 2016 Abstract: The Palmaris longus muscle can be absent unilateral or bilateral in about 22.4% of human beings. The aim of this study is to investigate whether the presence of the Palmaris longus muscle is associated with an advantage to handgrip in elite tennis players compared to recreational athletes. Sixty people participated in this study, thirty elite tennis players and thirty recreational athletes. The presence of the Palmaris longus muscle was first assessed using different tests. Grip strength and fatigue resistance were measured by an electronic hand dynamometer. Proprioception was registered by the Flock of Birds electromagnetic tracking system. Three tests were set up for measuring proprioception: joint position sense, kinesthesia, and joint motion sense. Several hand movements were conducted with the aim to correctly reposition the joint angle. Results demonstrate a higher presence of the Palmaris longus muscle in elite tennis players, but this was not significant. -

Nerve Entrapment Syndromes 1091
1090 Part VIII Septic and Nontraumatic Conditions may present with well-defi ned symptoms of ulnar CHAPTER 80 nerve compression at the elbow; electrical studies, however, may have normal results in the ulnar nerve but reveal changes of carpal tunnel syndrome (which Nerve Entrapment may be either subclinical or less symptomatic to the patient). Post-traumatic thickening of the brachial Syndromes fascia in the distal arm can produce a simultaneous median and lateral antebrachial nerve compression. When more than one nerve is suspected in the neural Robert J. Spinner compression process, a more proximal lesion such as the brachial plexus, must be ruled out as the site of the pathologic process. 2. A nerve can be compressed at more than one level; INTRODUCTION that is, a “double crush” lesion may exist. This most commonly occurs at the neck and the wrist but can The diagnosis of a nerve entrapment lesion arising at also occur at other locations such as the thoracic the elbow can be relatively straightforward if the history, outlet and the cubital tunnel. physical examination, electromyographic (EMG), and 3. Two separate neurologic processes may coexist. For imaging studies, when indicated, all confi rm the diagno- example, a patient who is wheelchair-bound from a sis and the localization of the lesion.12,32,47,87,93,138 However, syrinx may develop hand atrophy, which represents when the history and physical examination do not cor- new bilateral ulnar nerve compression rather than respond or the electrophysiologic or imaging studies do progression of the syrinx. Thus, on occasion, it is nec- not support a specifi c clinical diagnosis, then problems essary to direct one’s conservative or surgical atten- can arise. -

The Bicipital Aponeurosis
Surg Radiol Anat DOI 10.1007/s00276-017-1885-0 ORIGINAL ARTICLE Ultrasound visualization of an underestimated structure: the bicipital aponeurosis 1 1 1 M. Konschake • H. Stofferin • B. Moriggl Received: 15 February 2017 / Accepted: 31 May 2017 Ó The Author(s) 2017. This article is an open access publication Abstract the BA. Therefore, we suggest additional BA scanning during Purpose We established a detailed sonographic approach to clinical examinations of several pathologies, not only for BA the bicipital aponeurosis (BA), because different pathologies augmentation procedures in distal biceps tendon tears. of this, sometimes underestimated, structure are associated with vascular, neural and muscular lesions; emphasizing its Keywords Bicipital aponeurosis Á Lacertus fibrosus Á further implementation in routine clinical examinations. Biceps brachii muscle Á Ultrasonography Methods The BA of 100 volunteers, in sitting position with the elbow lying on a suitable table, was investigated. Patients were aged between 18 and 28 with no history of Introduction distal biceps injury. Examination was performed using an 18–6 MHz linear transducer (LA435; system MyLab25 by The biceps brachii muscle (BM) is attached distally to the Esaote, Genoa, Italy) utilizing the highest frequency, radial tuberosity via the strong biceps tendon (BT) and to scanned in two planes (longitudinal and transverse view). the antebrachial fascia via the bicipital aponeurosis (BA), In each proband, scanning was done with and without also known as lacertus fibrosus. As previously described, isometric contraction of the biceps brachii muscle. the BT consists of two distinct portions separated by an Results The BA was characterized by two clearly distin- endotenon septum and surrounded by a common paratenon, guishable white lines enveloping a hypoechoic band.