Granulated Polyhalite Fertilizer Caking Propensity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineralogy of the Martian Surface
EA42CH14-Ehlmann ARI 30 April 2014 7:21 Mineralogy of the Martian Surface Bethany L. Ehlmann1,2 and Christopher S. Edwards1 1Division of Geological & Planetary Sciences, California Institute of Technology, Pasadena, California 91125; email: [email protected], [email protected] 2Jet Propulsion Laboratory, California Institute of Technology, Pasadena, California 91109 Annu. Rev. Earth Planet. Sci. 2014. 42:291–315 Keywords First published online as a Review in Advance on Mars, composition, mineralogy, infrared spectroscopy, igneous processes, February 21, 2014 aqueous alteration The Annual Review of Earth and Planetary Sciences is online at earth.annualreviews.org Abstract This article’s doi: The past fifteen years of orbital infrared spectroscopy and in situ exploration 10.1146/annurev-earth-060313-055024 have led to a new understanding of the composition and history of Mars. Copyright c 2014 by Annual Reviews. Globally, Mars has a basaltic upper crust with regionally variable quanti- by California Institute of Technology on 06/09/14. For personal use only. All rights reserved ties of plagioclase, pyroxene, and olivine associated with distinctive terrains. Enrichments in olivine (>20%) are found around the largest basins and Annu. Rev. Earth Planet. Sci. 2014.42:291-315. Downloaded from www.annualreviews.org within late Noachian–early Hesperian lavas. Alkali volcanics are also locally present, pointing to regional differences in igneous processes. Many ma- terials from ancient Mars bear the mineralogic fingerprints of interaction with water. Clay minerals, found in exposures of Noachian crust across the globe, preserve widespread evidence for early weathering, hydrothermal, and diagenetic aqueous environments. Noachian and Hesperian sediments include paleolake deposits with clays, carbonates, sulfates, and chlorides that are more localized in extent. -

Evaporites Deposits Evaporites Are Formed in Closed Or Semi-Closed Basins Where Evaporation Exceeds Precipitation (+ Runoff)
Evaporites deposits Evaporites are formed in closed or semi-closed basins where evaporation exceeds precipitation (+ runoff). Rogers Dry Lake, Mojave Desert, California The Salar de Uyuni Lake in Bolivia (more than 9,000 km2) is the largest salt playa in the world. The salar (salt pan) has about a meter of briny water (blue). In addition to sodium chloride, NaCl (rock salt), and calcium sulfate, CaSO4, the lake also contains lithium chloride, LiCl, making this the biggest source of lithium in the world. http://eps.mcgill.ca/~courses/c542/ 1/42 Chemical Fractionation and the Chemical Divide As a result of evaporation, chemical fractionation takes place between seawater and the remaining concentrated brines. The fractionation can be accounted for by a variety of mechanisms: 1) Mineral precipitation 2) Selective dissolution of efflorescent crusts and sediment coatings 3) Exchange and sorption on active surfaces 4) Degassing 5) Redox reactions Mineral precipitation is the most important and the one that can most easily be modeled. The basic assumption of the model is that minerals will precipitate as the solution becomes saturated with respect to a solid phase. In other words, precipitation occurs when the ion activity product of the solution becomes equal to the solubility constant of the mineral and remains constant upon further evaporation. The fate of seawater constituents upon mineral precipitation rests on the concept of the chemical divide. 2/42 Chemical Divides: Branching points along the path of solution evolution 3/42 Brine evolution and chemical divides 2+ 2- If we monitor the evaporation of a dilute Ca and SO4 solution, their concentrations will increase until we reach gypsum saturation. -

The Mineralogy of the British Permian Evaporites
460 The mineralogy of the British Permian evaporites By F. H. STEWART Department of Geology, University of Edinburgh Summary. The occurrence of the minerals is reviewed. Consideration of their genesis leads to a tentative list of primary minerals, and of early diagenetie, later diagenetic and geothermal metamorphic, and late near-surface changes. N 1943 Professor Tilley recorded the occurrence of po]yhalite in the I rocks of the D'Arcy Exploration Company's E.2 borehole at Aislaby, near Whitby; this was the first published record of a potassium-bearing salt in British evaporites. The occurrence of rocks containing sylvine (Lees and Taitt, 1946) prompted further exploration by Imperial Chemical Industries and Fisons Ltd. after the war, and this showed the presence of large deposits of potassium ores in the Whitby region. There is now a considerable literature related to these, and it seems timely to give a short review of the mineralogy of the British Permian evaporites. The writer is most grateful to Fisons Ltd. who have kindly allowed him to use unpublished information from his study of their boreholes F.1 at Robin Hood's Bay, F.2 at Staintondale, F.3 at Little Beck, and F.4 at Hawsker, in the Whitby Scarborough district. The position of these and the other boreholes mentioned in this paper can be found in the map of Dunham (1960). Dist~'ibution of the minerals The evaporites occur in two principal areas east and west of the Pennine Hills. In the western area (the Vale of Eden, west Cumberland, and the Furness district of Lancashire), where four evaporite beds have been recognized (Hollingworth, 1942), the mineralogy is relatively simple; carbonates, anhydrite, and gypsum are the main constituents. -

Polyhalite K2ca2mg(SO4)4 • 2H2O C 2001-2005 Mineral Data Publishing, Version 1
Polyhalite K2Ca2Mg(SO4)4 • 2H2O c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Triclinic, pseudo-orthorhombic. Point Group: 1. Rare crystals, tabular {010}, or elongated along [001], to 2 mm; complex development, with 28 forms recorded; fibrous, foliated, massive. Twinning: Common on {010}, {100}, characteristically polysynthetic. Physical Properties: Cleavage: On {101}, perfect; on {010}, a parting. Hardness = 3.5 D(meas.) = 2.78 D(calc.) = 2.76 Soluble in H2O, with precipitation of gypsum and perhaps syngenite. Optical Properties: Transparent. Color: Colorless, white, gray; commonly salmon-pink to brick-red from included iron oxide; colorless in transmitted light. Luster: Vitreous to resinous. Optical Class: Biaxial (–). α = 1.547–1.548 β = 1.560–1.562 γ = 1.567 2V(meas.) = 62◦–70◦ Cell Data: Space Group: P 1. a = 11.689 b = 16.332 c = 7.598 α =91.65◦ β =90◦ γ =91.9◦ Z=4 X-ray Powder Pattern: Netherlands. (ICDD 21-982). 3.18 (100), 2.913 (25), 2.890 (25), 2.849 (25), 2.945 (20), 6.00 (14), 2.904 (14) Chemistry: (1) (2) SO3 52.40 53.12 MgO 6.48 6.68 CaO 18.75 18.60 K2O 15.66 15.62 H2O 6.07 5.98 rem. 0.27 Total 99.63 100.00 • (1) McDowell Well, Glasscock Co., Texas, USA. (2) K2Ca2Mg(SO4)4 2H2O. Occurrence: Common in marine salt deposits, where it may constitute the major component, present in billion-ton amounts; diagenetic in playa muds, formed from gypsum in the presence of Mg–K-rich solutions; rare as a fumarolic sublimate. -

Crystallization of Kainite from Solutions in Syste
ineering ng & E P l r a o c i c e m s e s Journal of h T C e f c h o Kostiv and Basystiuk, J Chem Eng Process Technol 2016, 7:3 l ISSN: 2157-7048 n a o n l o r g u y o J Chemical Engineering & Process Technology DOI: 10.4172/2157-7048.1000298 Research Article Article OpenOpen Access Access + 2+ + - 2- Crystallization of Kainite from Solutions in System K , Mg , Na // Cl , SO4 -Н2О Kostiv IY1 and Yа І Basystiuk2* 1State Enterprise Scientific - Research Gallurgi Institute 5a, Fabrichna Str., 76000 Kalush, Ukraine 2Precarpathian National University named after V. Stephanyk 57, Shevchenko Str., 76025 Ivano-Frankivsk, Ukraine Abstract In isothermal conditions have been studied the influence of system solution sea salts during their evaporation and crystallization to composition of received liquid and solid phases. It is shown that evaporation of the solution leading 2- to its supersaturation by sulfate salts. In the liquid phase before crystallization of kainite concentration of SO4 ions increases to 10% and above. Through this probability of formation of crystals increases and the result of evaporation 2- is the formation of finely dispersed kainite. During crystallization of kainite concentration of SO4 in the liquid phase 2- 2+ decreases rapidly. Decreasing its intensity increases with increasing value k=E SO4 : E Mg of initial solution. For 2- the degree of evaporation of 20.0% and 31.0% concentration of SO4 in evaporated solution on the value of k does not change. Most forms of potassium salts in the solid phase for the value k=0.7573 and reaches a maximum value at 82% evaporation rate of 31%. -

Mechanism and Kinetics of Dehydration of Epsomite Crystals Formed in the Presence of Organic Additives
J. Phys. Chem. B 2007, 111, 41-52 41 Mechanism and Kinetics of Dehydration of Epsomite Crystals Formed in the Presence of Organic Additives Encarnacio´n Ruiz-Agudo,† J. Daniel Martı´n-Ramos,‡ and Carlos Rodriguez-Navarro* Departamento de Mineralogı´a y Petrologı´a, UniVersidad de Granada, FuentenueVa s/n, 18002 Granada, Spain ReceiVed: July 14, 2006; In Final Form: October 10, 2006 The thermal dehydration of epsomite (MgSO4‚7H2O) crystals grown in the presence and absence of organic additives (phosphonates, carboxylic acids, and polyacrylic acid derivatives) was studied by means of thermogravimetry (TG), differential scanning calorimetry (DSC), X-ray thermodiffraction (XRTD), and environmental scanning electron microscopy (ESEM). In situ XRTD analyses (in air, 30% relative humidity) show an epsomite f hexahydrite (MgSO4‚6H2O) transition at 25-38 °C, followed by formation of amorphous phase(s) at T > 43-48 °C, and MgSO4 crystallization at ∼300 °C. Kinetic parameters (ER and A) were determined for the main dehydration step (25-160 °C), which corresponds to a MgSO4‚7H2O f MgSO4‚ H2O transition, by applying two isoconversional methods to nonisothermal TG data obtained at different heating rates (â ) 1, 3, and 5 K‚min-1). In situ, hot-stage ESEM observations of the thermal dehydration of epsomite crystals are consistent with the nonisothermal kinetic study and, along with XRTD results, allow us to propose a dehydration mechanism which includes an early nucleation and growth event, followed by the advancement of the reaction interface (3D phase boundary reaction). Both ER and A values increase in the presence of the most effective crystallization inhibitors tested. -

Stability of Magnesium Sulfate Minerals in Martian Environments
Lunar and Planetary Science XXXVI (2005) 2290.pdf STABILITY OF MAGNESIUM SULFATE MINERALS IN MARTIAN ENVIRONMENTS. G.M. Marion1 and J.S. Kargel2, 1Desert Research Institute, 2215 Raggio Parkway, Reno, NV 89512, [email protected], 2U.S. Geological Survey, 2255 N. Gemini Dr., Flagstaff, AZ 86001, [email protected]. Introduction: Viking Lander, Pathfinder, and –3.6°C for a pure MgSO4-H2O system. In this system, Mars Exploration Rover missions to Mars have found the lower hydrates, hexahydrite and kieserite, only abundant sulfur in surface soils and rocks, and the best form at higher temperatures (48°C and 69°C, respec- indications are that magnesium sulfates are among the tively). key hosts [1-6]. At Meridiani Planum, MgSO4 salts The presence of other constituents in ternary (or constitute 15 to 40 wt.% of sedimentary rocks [3-5,7]. higher) systems can lower the temperatures at which Additional S is hosted by gypsum and jarosite. Reflec- lower hydrates are stable. For example, hexahydrite is tance and thermal emission spectroscopy is consistent stable at 25°C in a MgSO4-MgCl2-H2O system at 3.8 with the presence of kieserite (MgSO4•H2O) and ep- m MgCl2 [12]. This requires high chloride concentra- somite (MgSO4•7H2O) [3]. Theoretically, the tions that may occur in Martian environments that dodecahydrate (MgSO4•12H2O) should also have have previously undergone extensive sulfate precipita- precipitated [8]. tion and chloride concentration due to extreme freez- We first examine theoretically which MgSO4 min- ing or desiccating conditions [3-5]. erals should have precipitated on Mars, and then how Based on the Rover mission to Meridiani Planum, dehydration might have altered these minerals. -

High Temperature Sulfate Minerals Forming on the Burning Coal Dumps from Upper Silesia, Poland
minerals Article High Temperature Sulfate Minerals Forming on the Burning Coal Dumps from Upper Silesia, Poland Jan Parafiniuk * and Rafał Siuda Faculty of Geology, University of Warsaw, Zwirki˙ i Wigury 93, 02-089 Warszawa, Poland; [email protected] * Correspondence: j.parafi[email protected] Abstract: The subject of this work is the assemblage of anhydrous sulfate minerals formed on burning coal-heaps. Three burning heaps located in the Upper Silesian coal basin in Czerwionka-Leszczyny, Radlin and Rydułtowy near Rybnik were selected for the research. The occurrence of godovikovite, millosevichite, steklite and an unnamed MgSO4, sometimes accompanied by subordinate admixtures of mikasaite, sabieite, efremovite, langbeinite and aphthitalite has been recorded from these locations. Occasionally they form monomineral aggregates, but usually occur as mixtures practically impossible to separate. The minerals form microcrystalline masses with a characteristic vesicular structure resembling a solidified foam or pumice. The sulfates crystallize from hot fire gases, similar to high temperature volcanic exhalations. The gases transport volatile components from the center of the fire but their chemical compositions are not yet known. Their cooling in the near-surface part of the heap results in condensation from the vapors as viscous liquid mass, from which the investigated minerals then crystallize. Their crystallization temperatures can be estimated from direct measurements of the temperatures of sulfate accumulation in the burning dumps and studies of their thermal ◦ decomposition. Millosevichite and steklite crystallize in the temperature range of 510–650 C, MgSO4 Citation: Parafiniuk, J.; Siuda, R. forms at 510–600 ◦C and godovikovite in the slightly lower range of 280–450 (546) ◦C. -
![The Paragenesis of Sylvine, Carnallite, Polyhalite, and ]Cieserite in Eskdale Borings Nos](https://docslib.b-cdn.net/cover/6717/the-paragenesis-of-sylvine-carnallite-polyhalite-and-cieserite-in-eskdale-borings-nos-1696717.webp)
The Paragenesis of Sylvine, Carnallite, Polyhalite, and ]Cieserite in Eskdale Borings Nos
667 The paragenesis of sylvine, carnallite, polyhalite, and ]cieserite in Eskdale borings nos. 3, 4, and 6, north-east Yorkshire. (With Plate XXV.) G. ARMSTRO~O, B.Se., F.G.S., K. C. DUNHAM, D.Sc., S.D., F.G.S., C. 0. HAI~VEY, B.Sc., A.R.C.S., F.R.I.C., P. A. SABINe, Ph.D., A.R.C.S., F.G.S., and W. F. WATERS, B.Sc., F.R.I.C. Geological Survey of Great Britain. [Read March 8, 1951.] INTRODUCTION. HE confirmation of the presence of potassium salts in the Permian T evaporites beneath the Whitby district, first found in the D'Arey Exploration Co.'s Eskdale no. 2 (Aislaby) boring (G. M. Lees and A. H. Taitt, 1945), and the discovery by Imperial Chemical Industries in their Eskdale no. 3 (Sleights), no. 4 (Sneaton), and no. 6 (Upgang) borings that these salts exist in workable quality and quantity, has been recorded in a recent historic paper by Dr. A. Fleck (1950). The successful outcome of this boring campaign may well lead to the outstanding development of the present century in economic mineralogy in Britain. In the course of the exploration the Geological Survey has examined in detail the cores obtained, and every facility has been granted by Imperial Chemical Industries for the removal of specimens from the cores for preservation in the national collection at the Geological Museum. The Petrographical Department of the Geological Survey has, on its side, provided reports on the mineralogy of the cores. The present brief summary of these reports, with some additional chemical, X-ray, and petrographical data, is published by permission of the Director of the Geological Survey and with the approval of Imperial Chemical Industries, Limited. -
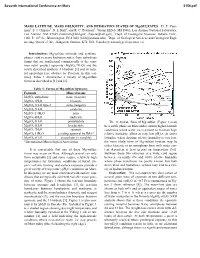
MARS LATITUDE, MARS OBLIQUITY, and HYDRATION STATES of Mg-SULFATES
Seventh International Conference on Mars 3156.pdf MARS LATITUDE, MARS OBLIQUITY, AND HYDRATION STATES OF Mg-SULFATES. D. T. Vani- man1, S. J. Chipera1, D. L Bish2, and R. C. Peterson3, 1Group EES-6, MS D462, Los Alamos National Laboratory, Los Alamos, NM 87545 ([email protected]; [email protected]), 2Dept. of Geological Sciences, Indiana Univ., 1001 E. 10th St., Bloomington, IN 47405 ([email protected]), 3Dept. of Geological Sciences and Geological Engi- neering, Queen’s Univ., Kingston, Ontario, K7L 3N6, Canada ([email protected]). Introduction: Mg-sulfate minerals and synthetic phases exist in many hydration states, from anhydrous forms that are synthesized commercially to the com- mon retail product epsomite (MgSO4·7H2O) and the newly described synthetic 11-hydrate [1] and its natu- ral equivalent [see abstract by Peterson in this vol- ume]. Table 1 summarizes a variety of Mg-sulfate forms as described in [1] and [2]. Table 1: Forms of Mg-sulfate hydrates Formula Mineral name MgSO4 anhydrous none (reagent) MgSO4·1H2O kieserite MgSO4·1H2O type 2 none (reagent) MgSO4·2H2O sanderite MgSO4·2.4H2O none MgSO ·4H O starkeyite 4 2 MgSO4·5H2O pentahydrite The 11-hydrate form of Mg-sulfate (Figure 1) may MgSO4·6H2O hexahydrite be a stable phase on Mars under current higher-latitude MgSO4·7H2O epsomite conditions where water ice is present to maintain high MgSO4·11H2O pending approval by IMA* relative humidity, albeit at very low pH2O. At lower MgSO4·nH2O amorphous (n variable) latitudes, where daytime relative humidity is very low, *International Mineralogical Association the more likely form of Mg-sulfate hydrate may be either kieserite or an amorphous form with water con- It is conceivable that any of these Mg-sulfate tent dependent at least in part on temperature [5,6]. -

Co2 Storage As Carbonate Minerals
CO2 STORAGE AS CARBONATE MINERALS Report Number PH3/17 February 2000 This document has been prepared for the Executive Committee of the Programme. It is not a publication of the Operating Agent, International Energy Agency or its Secretariat. Title: CO2 storage as carbonate minerals Reference number: PH3/17 Date issued: February 2000 Other remarks: Introduction This study is an assessment of above-ground processes that would bind CO2 as a mineral carbonate. For example, a number of people have suggested converting naturally occurring mineral oxides to carbonates by reaction with CO2 captured at a power station. The mineral carbonate would be stored indefinitely. If carbonate storage were to be used, it could be a safe store for CO2 captured during the generation of power from fossil fuels. Such a process would have the attraction of ‘locking-up’ CO2 in a chemical that occurs naturally and is widely distributed, thus avoiding many of the environmental and safety concerns expressed about other storage options. Potentially suitable minerals for CO2 sequestration are, in general, complex silicates that originated deep within the earth’s mantle. There are huge quantities of such rocks and, over geological time- scales, they naturally ‘weather’ to a carbonate by reaction with atmospheric CO2. However, the carbonation reaction is extremely slow at normal temperatures and pressures. In addition, the mass of rock required is several times the mass of CO2 stored. In principle, these disadvantages could be minimised by use of large-scale processes similar to those used in mineral extraction industries where large quantities of materials are routinely processed to recover a relatively small quantity of product. -

THE MINERALOGICAL MAGAZINE Alqd JOURNAL of the MINERALOGICAL SOCIETY
THE MINERALOGICAL MAGAZINE AlqD JOURNAL OF THE MINERALOGICAL SOCIETY No. 206 September, 1949 Vol. XXVIII The petrology of the evaporites of the Eskdale no. 2 boring, east Yorkshire. PART I. The lower evaporite bed. (With Plates XXIX-XXXIV.) By F. H. STEWART, B.Sc., Ph.D., F.G.S. Department of Geology, Science Laboratories, Durham, University of Durham. [Read March 31, 1949.] CO~TE~TS 2. Introduction and acknow- (6) Anhydrite-magnesitc- ledgements ...... 622 halite-talc-rock ... 643 II. Material examined ... 623 (7) Anhydrite-dolomite- III. General succession in the magnesite-rock ... 645 lower evaporite bed ... 625 VIL Polyhalite- bearing rocks : IV. The minerals: (1) Halite-anhydrite-poly- (l) Anhydrite ...... 626 halite -talc -rock 648 (2) Celestine ...... 627 (2) Development of poly~ (3) Dolomite ...... 628 halite in compact (4) Gypsum ...... 628 anhydrite-dolomite- (5) Halite ......... 629 rock ......... 651 (6) Magnesite ...... 630 (3) Polyhalite-bearing (7) Polyhalite ...... 631 rocks with pseudo- (8) Pyrite ......... 632 morphous structure (9) Quartz ......... 632 after gypsum ... 652 (10) Native Sulphur ... 632 (4) Rocks consisting almost (11) Talc ......... 633 entirely of polyhal- V. Halite-anhydrite-rocks ... 633 ite ... 655 VI. Anhydrite-carbonate-rocks: {5) Anhydrite porphyro- (1) Anhydrite-dolomite- blasts in polyhalite- rock 637 bearing rocks ... 656 (2) Brecciated anhydrite- (6) Chemical composition 656 dolomite-rock ... 639 VIII. Origin of the polyhalite- (3) Anhydrite-dolomite- bearing rocks ...... 657 rock with coarse fibro- IX. The relation between mag- radiate anhydrite ... 641 nesite and dolomite ... 663 (4) Compact aahydrite- X. The distribution and origin magnesite-rock ... of tale 665 (5) Coarse anhydrite-mag- 642 l XL Summary and conclusions 669 nesite-halite-rock ..