Effector Memory T Cells + Regulation of IL-17 in Human CCR6
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effector CD4 T-Cell Transition to Memory Requires Late Cognate Interactions That Induce Autocrine IL-2
ARTICLE Received 3 Jun 2014 | Accepted 24 Sep 2014 | Published 5 Nov 2014 DOI: 10.1038/ncomms6377 Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2 K. Kai McKinstry1,*, Tara M. Strutt1,*, Bianca Bautista1, Wenliang Zhang1, Yi Kuang1, Andrea M. Cooper2 & Susan L. Swain1 It is unclear how CD4 T-cell memory formation is regulated following pathogen challenge, and when critical mechanisms act to determine effector T-cell fate. Here, we report that following influenza infection most effectors require signals from major histocompatibility complex class II molecules and CD70 during a late window well after initial priming to become memory. During this timeframe, effector cells must produce IL-2 or be exposed to high levels of paracrine or exogenously added IL-2 to survive an otherwise rapid default contraction phase. Late IL-2 promotes survival through acute downregulation of apoptotic pathways in effector T cells and by permanently upregulating their IL-7 receptor expression, enabling IL-7 to sustain them as memory T cells. This new paradigm defines a late checkpoint during the effector phase at which cognate interactions direct CD4 T-cell memory generation. 1 Department of Pathology, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, Massachusetts 01655, USA. 2 Trudeau Institute, 154 Algonquin Avenue, Saranac Lake, New York 12983, USA. * These authors contributed equally to this work. Correspondence and requests for materials should be addressed to K.K.M. (email: [email protected]). NATURE COMMUNICATIONS | 5:5377 | DOI: 10.1038/ncomms6377 | www.nature.com/naturecommunications 1 & 2014 Macmillan Publishers Limited. -

Memory T Cells + Maintenance of CD8 the Dual Role of IL-2 in the Generation
The Dual Role of IL-2 in the Generation and Maintenance of CD8 + Memory T Cells Zhenhua Dai, Bogumila T. Konieczny and Fadi G. Lakkis This information is current as J Immunol 2000; 165:3031-3036; ; of September 30, 2021. doi: 10.4049/jimmunol.165.6.3031 http://www.jimmunol.org/content/165/6/3031 Downloaded from References This article cites 31 articles, 17 of which you can access for free at: http://www.jimmunol.org/content/165/6/3031.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on September 30, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2000 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Dual Role of IL-2 in the Generation and Maintenance of CD8؉ Memory T Cells1 Zhenhua Dai, Bogumila T. Konieczny, and Fadi G. Lakkis2 The mechanisms responsible for the generation and maintenance of T cell memory are unclear. In this study, we tested the role of IL-2 in allospecific CD8؉ T cell memory by analyzing the long-term survival, phenotype, and functional characteristics of IL-2-replete (IL-2؉/؉) and IL-2-deficient (IL-2؊/؊) CD8؉ TCR-transgenic lymphocytes in an adoptive transfer model. -

Vaccine Immunology Claire-Anne Siegrist
2 Vaccine Immunology Claire-Anne Siegrist To generate vaccine-mediated protection is a complex chal- non–antigen-specifc responses possibly leading to allergy, lenge. Currently available vaccines have largely been devel- autoimmunity, or even premature death—are being raised. oped empirically, with little or no understanding of how they Certain “off-targets effects” of vaccines have also been recog- activate the immune system. Their early protective effcacy is nized and call for studies to quantify their impact and identify primarily conferred by the induction of antigen-specifc anti- the mechanisms at play. The objective of this chapter is to bodies (Box 2.1). However, there is more to antibody- extract from the complex and rapidly evolving feld of immu- mediated protection than the peak of vaccine-induced nology the main concepts that are useful to better address antibody titers. The quality of such antibodies (e.g., their these important questions. avidity, specifcity, or neutralizing capacity) has been identi- fed as a determining factor in effcacy. Long-term protection HOW DO VACCINES MEDIATE PROTECTION? requires the persistence of vaccine antibodies above protective thresholds and/or the maintenance of immune memory cells Vaccines protect by inducing effector mechanisms (cells or capable of rapid and effective reactivation with subsequent molecules) capable of rapidly controlling replicating patho- microbial exposure. The determinants of immune memory gens or inactivating their toxic components. Vaccine-induced induction, as well as the relative contribution of persisting immune effectors (Table 2.1) are essentially antibodies— antibodies and of immune memory to protection against spe- produced by B lymphocytes—capable of binding specifcally cifc diseases, are essential parameters of long-term vaccine to a toxin or a pathogen.2 Other potential effectors are cyto- effcacy. -

Effector Memory T Cells + Regulation of IL-17 in Human CCR6
Regulation of IL-17 in Human CCR6+ Effector Memory T Cells Hong Liu and Christine Rohowsky-Kochan This information is current as J Immunol 2008; 180:7948-7957; ; of September 26, 2021. doi: 10.4049/jimmunol.180.12.7948 http://www.jimmunol.org/content/180/12/7948 Downloaded from References This article cites 25 articles, 9 of which you can access for free at: http://www.jimmunol.org/content/180/12/7948.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on September 26, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2008 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Regulation of IL-17 in Human CCR6؉ Effector Memory T Cells1 Hong Liu and Christine Rohowsky-Kochan2 IL-17–secreting T cells represent a distinct CD4؉ effector T cell lineage (Th17) that appears to be essential in the pathogenesis of numerous inflammatory and autoimmune diseases. Although extensively studied in the murine system, human Th17 cells have not been well characterized. -

Memory CD4 T Cell Subsets Are Kinetically Heterogeneous
RESEARCH ARTICLE Memory CD4 T cell subsets are kinetically heterogeneous and replenished from naive T cells at high levels Graeme Gossel1,2†§, Thea Hogan3†, Daniel Cownden1, Benedict Seddon3*‡, Andrew J Yates1*‡ 1Institute of Infection, Immunity and Inflammation, College of Medical, Veterinary & Life Sciences University of Glasgow, Glasgow, United Kingdom; 2Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, United States; 3Institute of Immunity and Transplantation, University College London, London, United Kingdom Abstract Characterising the longevity of immunological memory requires establishing the rules underlying the renewal and death of peripheral T cells. However, we lack knowledge of the population structure and how self-renewal and de novo influx contribute to the maintenance of *For correspondence: benedict. memory compartments. Here, we characterise the kinetics and structure of murine CD4 T cell [email protected] (BS); andrew. [email protected] (AJY) memory subsets by measuring the rates of influx of new cells and using detailed timecourses of DNA labelling that also distinguish the behaviour of recently divided and quiescent cells. We find † These authors contributed that both effector and central memory CD4 T cells comprise subpopulations with highly divergent equally to this work rates of turnover, and show that inflows of new cells sourced from the naive pool strongly impact ‡ These authors also contributed estimates of memory cell lifetimes and division rates. We also demonstrate that the maintenance of equally to this work CD4 T cell memory subsets in healthy mice is unexpectedly and strikingly reliant on this Present address: §Department replenishment. of Physics and Astronomy, DOI: 10.7554/eLife.23013.001 Hunter College, City University of New York, New York, United States Competing interests: The authors declare that no Introduction competing interests exist. -

Human T Cell Memory: a Dynamic View
vaccines Review Human T Cell Memory: A Dynamic View Derek C. Macallan 1,2,*, José A. M. Borghans 3 and Becca Asquith 4 1 Institute for Infection & Immunity, St George’s, University of London, Cranmer Terrace, London SW17 0RE, UK 2 St George’s University Hospitals NHS Foundation Trust, Blackshaw Road, London SW17 0QT, UK 3 Laboratory of Translational Immunology, Department of Immunology, University Medical Center Utrecht, P.O. Box 85090, 3508 AB Utrecht, The Netherlands; [email protected] 4 Department of Medicine, Imperial College London W2 1PG, UK; [email protected] * Correspondence: [email protected]; Tel.: +44-208-725-0283; Fax: +44-208-725-3487 Academic Editor: Stephen Todryk Received: 18 July 2016; Accepted: 17 January 2017; Published: 4 February 2017 Abstract: Long-term T cell-mediated protection depends upon the formation of a pool of memory cells to protect against future pathogen challenge. In this review we argue that looking at T cell memory from a dynamic viewpoint can help in understanding how memory populations are maintained following pathogen exposure or vaccination. For example, a dynamic view resolves the apparent paradox between the relatively short lifespans of individual memory cells and very long-lived immunological memory by focussing on the persistence of clonal populations, rather than individual cells. Clonal survival is achieved by balancing proliferation, death and differentiation rates within and between identifiable phenotypic pools; such pools correspond broadly to sequential stages in the linear differentiation pathway. Each pool has its own characteristic kinetics, but only when considered as a population; single cells exhibit considerable heterogeneity. -

Properties of Memory T Lymphocytes Isolated from the Mixed Leukocyte Reaction
Proc. Nati. Acad. Sci. USA Vol. 82, pp. 7686-7690, November 1985 Immunology Properties of memory T lymphocytes isolated from the mixed leukocyte reaction (dendritic celis/antigen-presenting cells/helper lymphocytes/macrophages) KAYO INABA*, SUMI KOIDE, AND RALPH M. STEINMANt The Rockefeller University and The Irvington House Institute, New York, NY 10021 Communicated by Zanvil A. Cohn, July 8, 1985 ABSTRACT During the primary mixed leukocyte reac- which we define as lymphoblasts that revert to smaller IL-2 tion, T lymphocytes of the lyt-2- helper subclass proliferate in unresponsive cells when the antigenic stimulus is removed. response to transplantation antigens on allogeneic dendritic We will describe a method for isolating memory cells from the cells. We have isolated populations of antigen-specific prolif- MLR and report several distinctive features in their response erating lymphoblasts and recultured them in fresh medium. to antigenic challenge. Within 2 days, the blasts become smaller in size, lose respon- siveness to T-cell growth factor or interleukin 2, but retain vigorous reactivity to the original alloantigen. Two new biologic MATERIALS AND METHODS properties of these "memory" lymphocytes have been noted. Cells. DC were derived from low-density spleen-adherent First, they primarily respond to alloantigen on dendritic cells, populations (10, 17). B cells were Sephadex G-10 nonadher- whereas freshly sensitized lymphoblasts react to allogeneic ent spleen cells that were treated with a-lyt-1 and athy-l and dendritic cells, macrophages, and B lymphocytes. Second, the complement to remove T lymphocytes (13). Macrophages memory lymphocytes quickly aggregate with dendritic cells were adherent resident peritoneal cells cultured 24 hr with that are either syngeneic or allogeneic, but not with B cells. -

T CELL MEMORY Efficient at Migrating to Inflamed Peripheral Tissues
RESEARCH HIGHLIGHTS T CELL MEMORY efficient at migrating to inflamed peripheral tissues. These data suggest that tissue-resident memory CD8+ T cells markedly outnumber those New insight on old-timers that are recirculating and that our current paradigms of memory T cell Memory T cells ensure that the recirculate between the blood and subsets require revision. immune response is more effective non-lymphoid tissues and respond Cui et al. explored how metabolic against re-infecting pathogens. Two rapidly to re-infecting pathogens. processes regulate longevity in mem- key distinctions between memory A further set of tissue-resident mem- ory CD8+ T cells. They compared + CD8 T cells and their naive counter- ory T (TRM) cells have been described gene expression profiles of naive, parts are crucial for this: first, mem- that are retained in non-lymphoid effector and memory CD8+ T cells ory T cells have distinct migratory tissues and do not recirculate. The and found that the glycerol channel patterns; and second, they survive for relative contribution of each subset to aquaporin 9 (AQP9) was selectively longer. New studies by Steinert et al. immune memory has been unclear; expressed by memory CD8+ T cells. and Cui et al. offer fresh insight into to address this, Steinert et al. devel- These cells were shown to upregulate both of these aspects of memory. oped a quantitative immunofluores- AQP9 in response to stimulation Memory CD8+ T cells have been cence microscopy (QIM) method. with interleukin‑7 (IL‑7) — and, to a divided into distinct subsets on the our current In a mouse model of lymphocytic lesser extent, in response to IL‑15 — basis of putative trafficking and func- paradigms choriomeningitis virus (LCMV) and deficiency of AQP9 impaired the tional properties. -

Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy
cancers Review Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy Vita Golubovskaya * and Lijun Wu * Promab Biotechnologies, 2600 Hilltop Drive, Suite 320, Richmond, CA 94803, USA * Correspondence: [email protected] (V.G.); [email protected] (L.W.); Tel.: +1-352-262-4846 (V.G.); +1-510-529-3021 (L.W.); Fax: +1-510-740-3625 (V.G. & L.W.) Academic Editor: Samuel C. Mok Received: 8 January 2016; Accepted: 10 March 2016; Published: 15 March 2016 Abstract: This review is focused on different subsets of T cells: CD4 and CD8, memory and effector functions, and their role in CAR-T therapy—-a cellular adoptive immunotherapy with T cells expressing chimeric antigen receptor. The CAR-T cells recognize tumor antigens and induce cytotoxic activities against tumor cells. Recently, differences in T cell functions and the role of memory and effector T cells were shown to be important in CAR-T cell immunotherapy. The CD4+ subsets (Th1, Th2, Th9, Th17, Th22, Treg, and Tfh) and CD8+ memory and effector subsets differ in extra-cellular (CD25, CD45RO, CD45RA, CCR-7, L-Selectin [CD62L], etc.); intracellular markers (FOXP3); epigenetic and genetic programs; and metabolic pathways (catabolic or anabolic); and these differences can be modulated to improve CAR-T therapy. In addition, CD4+ Treg cells suppress the efficacy of CAR-T cell therapy, and different approaches to overcome this suppression are discussed in this review. Thus, next-generation CAR-T immunotherapy can be improved, based on our knowledge of T cell subsets functions, differentiation, proliferation, and signaling pathways to generate more active CAR-T cells against tumors. -

Mechanism of Action of IL-7 and Its Potential Applications and Limitations in Cancer Immunotherapy
Int. J. Mol. Sci. 2015, 16, 10267-10280; doi:10.3390/ijms160510267 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Review Mechanism of Action of IL-7 and Its Potential Applications and Limitations in Cancer Immunotherapy Jianbao Gao 1, Lintao Zhao 1, Yisong Y. Wan 2,* and Bo Zhu 1,3,* 1 Institute of Cancer, Xinqiao Hospital, Third Military Medical University, Chongqing 400037, China; E-Mails: [email protected] (J.G.); [email protected] (L.Z.) 2 Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA 3 Biomedical Analysis Center, Third Military Medical University, Chongqing 400038, China * Authors to whom correspondence should be addressed; E-Mails: [email protected] (Y.Y.W.); [email protected] (B.Z.); Tel./Fax: +1-919-966-9728 (Y.Y.W); +86-23-6875-5626 (B.Z.). Academic Editor: Kamal D. Moudgil Received: 12 March 2015 / Accepted: 29 April 2015 / Published: 6 May 2015 Abstract: Interleukin-7 (IL-7) is a non-hematopoietic cell-derived cytokine with a central role in the adaptive immune system. It promotes lymphocyte development in the thymus and maintains survival of naive and memory T cell homeostasis in the periphery. Moreover, it is important for the organogenesis of lymph nodes (LN) and for the maintenance of activated T cells recruited into the secondary lymphoid organs (SLOs). The immune capacity of cancer patients is suppressed that is characterized by lower T cell counts, less effector immune cells infiltration, higher levels of exhausted effector cells and higher levels of immunosuppressive cytokines, such as transforming growth factor β (TGF-β). -

Expression of Chemokine Receptor CXCR3 on T Cells Affects the Balance Between Effector and Memory CD8 T-Cell Generation
Expression of chemokine receptor CXCR3 on T cells PNAS PLUS affects the balance between effector and memory CD8 T-cell generation Joyce K. Hua,b, Takashi Kagaria,1, Jonathan M. Clingana,b, and Mehrdad Matloubiana,2 aDivision of Rheumatology, Department of Medicine, and Rosalind Russell Medical Research Center for Arthritis, and bGraduate Program in Biomedical Sciences, University of California, San Francisco, CA 94143 AUTHOR SUMMARY Killer T cells, also known as CD8 T cells, Localization terminally differentiated effector cells are essential for protection against viru- WT CD8 T cells CXCR3 KO CD8 T cells and long-lived memory cells. ses and many other intracellular patho- Naive CD8 T cells, mature but inactive gens. In response to an infection, Red pulp killer T cells that have not yet encoun- pathogen-specific CD8 T cells rapidly tered their respective antigens, continu- zone proliferate and differentiate into a het- Marginal ously circulate through peripheral erogeneous population of effector cells, lymphoid organs, such as the spleen and the majority of which undergo apoptosis lymph nodes, in search of an antigen after the infection is controlled. How- capable of activating their pathogen- ever, a small fraction of the effector cells fighting functions. Within these lymphoid survive, becoming self-renewing and cell zone T tissues, naive CD8 T cells are mostly lo- long-lived memory T cells that provide calized in so-called “T zones” through AAntigenntigen WT effector CCD8D8 T celcelll B cecellll rapid protection against subsequent the binding of homeostatic chemokines MMUNOLOGY CXCL9CXCL9 CXCR3CXCR3 KOKO effector CD8CD8 T cell challenges from the same pathogen. -
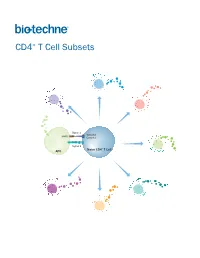
CD4+ T Cell Subsets
CD4+ T Cell Subsets Th2 Th1 Th9 Signal 1 TCR-CD3 MHCII Complex Th17 Signal 2 Naïve CD4+ T Cell APC Treg Th22 Tfh CD4+ T Cell Subsets CD4+ T cells play a central role in directing adaptive immune responses against invading microbial pathogens. Naïve CD4+ T cells differentiate into more specialized subsets following T cell activation, which requires recognition of the antigenic peptide/major histocompatibility complex (MHC) by the T cell receptor (Signal 1), along with antigen-independent co-stimulation (Signal 2). Differentiation into a specific CD4+ T cell subset depends on the antigen, the strength of the TCR signal, and cytokines present in the surrounding extracellular environment. The different CD4+ T helper cell subsets that have been identified include T helper 1 (Th1), Th2, Th9, Th17, and Th22 cells, along with follicular helper T (Tfh) cells, and regulatory T cells (Tregs). Each of these subsets expresses unique combinations of cell surface receptors, transcription factors, and secreted cytokines. While Tfh cells are primarily involved in promoting the survival and proliferation of germinal center B cells and supporting germinal center development, the Th1, Th2, Th9, Th17, and Th22 subsets have all been found to be involved in host defense against specific microbial pathogens. The defined array of cytokines secreted by these effector T cell subsets contributes to both elimination of the foreign pathogen and activation of other immune cell types. The activities of these cells are balanced in part by a unique subpopulation of CD4+ T cells known as regulatory T cells (Tregs). Tregs specialize in maintaining immune homeostasis and self-tolerance, dampening inflammation, and preventing the development of autoimmune disease.