Human T Cell Memory: a Dynamic View
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary - Cellbiology
1 Glossary - Cellbiology Blotting: (Blot Analysis) Widely used biochemical technique for detecting the presence of specific macromolecules (proteins, mRNAs, or DNA sequences) in a mixture. A sample first is separated on an agarose or polyacrylamide gel usually under denaturing conditions; the separated components are transferred (blotting) to a nitrocellulose sheet, which is exposed to a radiolabeled molecule that specifically binds to the macromolecule of interest, and then subjected to autoradiography. Northern B.: mRNAs are detected with a complementary DNA; Southern B.: DNA restriction fragments are detected with complementary nucleotide sequences; Western B.: Proteins are detected by specific antibodies. Cell: The fundamental unit of living organisms. Cells are bounded by a lipid-containing plasma membrane, containing the central nucleus, and the cytoplasm. Cells are generally capable of independent reproduction. More complex cells like Eukaryotes have various compartments (organelles) where special tasks essential for the survival of the cell take place. Cytoplasm: Viscous contents of a cell that are contained within the plasma membrane but, in eukaryotic cells, outside the nucleus. The part of the cytoplasm not contained in any organelle is called the Cytosol. Cytoskeleton: (Gk. ) Three dimensional network of fibrous elements, allowing precisely regulated movements of cell parts, transport organelles, and help to maintain a cell’s shape. • Actin filament: (Microfilaments) Ubiquitous eukaryotic cytoskeletal proteins (one end is attached to the cell-cortex) of two “twisted“ actin monomers; are important in the structural support and movement of cells. Each actin filament (F-actin) consists of two strands of globular subunits (G-Actin) wrapped around each other to form a polarized unit (high ionic cytoplasm lead to the formation of AF, whereas low ion-concentration disassembles AF). -

Bacterial Cell Membrane
BACTERIAL CELL MEMBRANE Dr. Rakesh Sharda Department of Veterinary Microbiology NDVSU College of Veterinary Sc. & A.H., MHOW CYTOPLASMIC MEMBRANE ➢The cytoplasmic membrane, also called a cell membrane or plasma membrane, is about 7 nanometers (nm; 1/1,000,000,000 m) thick. ➢It lies internal to the cell wall and encloses the cytoplasm of the bacterium. ➢It is the most dynamic structure of a prokaryotic cell. Structure of cell membrane ➢The structure of bacterial plasma membrane is that of unit membrane, i.e., a fluid phospholipid bilayer, composed of phospholipids (40%) and peripheral and integral proteins (60%) molecules. ➢The phospholipids of bacterial cell membranes do not contain sterols as in eukaryotes, but instead consist of saturated or monounsaturated fatty acids (rarely, polyunsaturated fatty acids). ➢Many bacteria contain sterol-like molecules called hopanoids. ➢The hopanoids most likely stabilize the bacterial cytoplasmic membrane. ➢The phospholipids are amphoteric molecules with a polar hydrophilic glycerol "head" attached via an ester bond to two non-polar hydrophobic fatty acid tails. ➢The phospholipid bilayer is arranged such that the polar ends of the molecules form the outermost and innermost surface of the membrane while the non-polar ends form the center of the membrane Fluid mosaic model ➢The plasma membrane contains proteins, sugars, and other lipids in addition to the phospholipids. ➢The model that describes the arrangement of these substances in lipid bilayer is called the fluid mosaic model ➢Dispersed within the bilayer are various structural and enzymatic proteins, which carry out most membrane functions. ➢Some membrane proteins are located and function on one side or another of the membrane (peripheral proteins). -

The TNF-Family Cytokine TL1A Drives IL-13-Dependent Small Intestinal Inflammation
ARTICLES nature publishing group See ARTICLE page 186 The TNF-family cytokine TL1A drives IL-13-dependent small intestinal inflammation F M e y l a n 1 , 6 , Y - J S o n g 1 , 6 , I F u s s 2 , S V i l l a r r e a l 1 , E K a h l e 1 , I - J M a l m 1 , K A c h a r y a 1 , H L R a m o s 3 , L L o 4 , M M M e n t i n k - K a n e 5 , T A W y n n 5 , T - S M i g o n e 4 , W S t r o b e r 2 a n d R M S i e g e l 1 The tumor necrosis factor (TNF)-family cytokine TL1A (TNFSF15) costimulates T cells through its receptor DR3 (TNFRSF25) and is required for autoimmune pathology driven by diverse T-cell subsets. TL1A has been linked to human inflammatory bowel disease (IBD), but its pathogenic role is not known. We generated transgenic mice that constitutively express TL1A in T cells or dendritic cells. These mice spontaneously develop IL-13-dependent inflammatory small bowel pathology that strikingly resembles the intestinal response to nematode infections. These changes were dependent on the presence of a polyclonal T-cell receptor (TCR) repertoire, suggesting that they are driven by components in the intestinal flora. Forkhead box P3 (FoxP3)-positive regulatory T cells (Tregs) were present in increased numbers despite the fact that TL1A suppresses the generation of inducible Tregs. -

Standard 2: CELL BIOLOGY – REVIEW of BASICS
Standard 2: CELL BIOLOGY – REVIEW OF BASICS CELL PART OR TYPE OF CELL WHERE FOUND WHAT DOES IT FUNCTION: MISCELLANEOUS ORGANELLE Prokaryotic cell Plant cell LOOK LIKE: Job it does in INFORMATION: things Eukaryotic cell Animal cell Describe or Draw the cell such as color, what it is Both Both made of, size, etc. plasma/cell See diagram Holds cell together Phospholipid bilayer with membrane both both Regulates what goes proteins in/out of cell Semipermeable cytoplasm both Clear thick jelly- Supports/protects both like material in cell cell organelles See diagram Control center nucleus eukaryotic both Contains DNA See diagram Where proteins are ribosome both both made See diagram Process proteins Golgi complex eukaryotic both that go to other /apparatus parts of cell Membrane-bound Digests materials lysosome eukaryotic animal sac of digestive within the cell enzymes Membrane-bound Stores water, food, One large one in plants vacuole eukaryotic both storage area waste and dissolved Many smaller ones in minerals animals endoplasmic Network of Transport materials Can be rough (with reticulum eukaryotic both membrane tubes throughout the cell ribosomes attached) or smooth (without ribosomes) See diagram Where cell respiration Called Powerhouse of cell mitochondria eukaryotic both occurs (releases Makes ATP from energy for cell to use) breaking down glucose See diagram Where photosynthesis Contains chlorophyll chloroplast eukaryotic plant takes place Converts light energy into chemical energy in glucose Some pro- and plant (also fungi Rigid structure -

Effector CD4 T-Cell Transition to Memory Requires Late Cognate Interactions That Induce Autocrine IL-2
ARTICLE Received 3 Jun 2014 | Accepted 24 Sep 2014 | Published 5 Nov 2014 DOI: 10.1038/ncomms6377 Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2 K. Kai McKinstry1,*, Tara M. Strutt1,*, Bianca Bautista1, Wenliang Zhang1, Yi Kuang1, Andrea M. Cooper2 & Susan L. Swain1 It is unclear how CD4 T-cell memory formation is regulated following pathogen challenge, and when critical mechanisms act to determine effector T-cell fate. Here, we report that following influenza infection most effectors require signals from major histocompatibility complex class II molecules and CD70 during a late window well after initial priming to become memory. During this timeframe, effector cells must produce IL-2 or be exposed to high levels of paracrine or exogenously added IL-2 to survive an otherwise rapid default contraction phase. Late IL-2 promotes survival through acute downregulation of apoptotic pathways in effector T cells and by permanently upregulating their IL-7 receptor expression, enabling IL-7 to sustain them as memory T cells. This new paradigm defines a late checkpoint during the effector phase at which cognate interactions direct CD4 T-cell memory generation. 1 Department of Pathology, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, Massachusetts 01655, USA. 2 Trudeau Institute, 154 Algonquin Avenue, Saranac Lake, New York 12983, USA. * These authors contributed equally to this work. Correspondence and requests for materials should be addressed to K.K.M. (email: [email protected]). NATURE COMMUNICATIONS | 5:5377 | DOI: 10.1038/ncomms6377 | www.nature.com/naturecommunications 1 & 2014 Macmillan Publishers Limited. -

Introduction to the Cell Cell History Cell Structures and Functions
Introduction to the cell cell history cell structures and functions CK-12 Foundation December 16, 2009 CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and distribution of high quality educational content that will serve both as core text as well as provide an adaptive environment for learning. Copyright ©2009 CK-12 Foundation This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License. To view a copy of this license, visit http://creativecommons.org/licenses/by-sa/3.0/us/ or send a letter to Creative Commons, 171 Second Street, Suite 300, San Francisco, California, 94105, USA. Contents 1 Cell structure and function dec 16 5 1.1 Lesson 3.1: Introduction to Cells .................................. 5 3 www.ck12.org www.ck12.org 4 Chapter 1 Cell structure and function dec 16 1.1 Lesson 3.1: Introduction to Cells Lesson Objectives • Identify the scientists that first observed cells. • Outline the importance of microscopes in the discovery of cells. • Summarize what the cell theory proposes. • Identify the limitations on cell size. • Identify the four parts common to all cells. • Compare prokaryotic and eukaryotic cells. Introduction Knowing the make up of cells and how cells work is necessary to all of the biological sciences. Learning about the similarities and differences between cell types is particularly important to the fields of cell biology and molecular biology. -

Immunity ≠ Immunological Memory
Immunity ≠ Immunological Memory Rolf Zinkernagel University of Zurich IMI Stakeholder Forum - 13 May 2013 - Brussels • Protective vaccines imitate co-evolution of infectious agents and hosts. • We cannot do better than evolution by using the same tools, only when introducing ‘new’ tools (antibiotics, antivirals, autoantibodies, behavioural changes) • Vaccines against solid tumours (carcinomas, sarcomas) and chronic persistent infections are theoretically not impossible but practically highly unlikely! Vaccines Not successful available Polio 1, 2, 3 TB bact. toxins Leprosy measles HIV Haem. infl. malaria (cholera) Neutr. / effector T cells opsonising antibodies antibodies igG (IgA) IMMUNITY • "innate resistance" > 95 % • Ab in eggs • protective memory via Ab (vaccines) • TB: no vaccine • autoimmunity > 30 y, female > male 5 : 1 • tumors > 30 years • Is what we measure biologically important ? (e.g. acad. memory: earlier + greater) CTL ELISA nAb Immunity Immunology Protection • ‚Memory‘ • Small pox • quicker - higher • Poliomeningitis • Measles • HIV, malaria - • Tuberculosis ± Antibody Memory after immunization with VSV a) neutralizing antibodies b) ELISA 12 12 ) 10 ) 10 2 3 8 8 40 log 10 log 6 - - 6 NP binding NP - 4 IND neutralizing 4 - IgG ( IgG ( 2 VSV 2 VSV 0 0 0 20 100 200 300 0 20 100 200 300 days after immunization days after immunization Why immunological Memory ? Protection (immunity) counts ! • Earlier + better • If first infection survived no need for memory • If first infection kills no need for memory • All vaccines that -
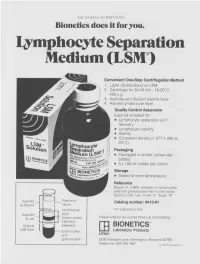
Lymphocyte Separation Medium (LSM
THE JOURNAL OF IMMUNOLOGY Bionetics does it for you. Lymphocyte Separation Medium (LSM wenient One-Step Centrifugation Method _ayer diluted blood on LSM. 2,entrifuge for 30-40 min., 18-20°C, ~.00 x g. ~,spirate and discard plasma layer -larvest lymphocyte layer. Quality Control Assurance Each lot is tested for: • Lymphocyte separation and recovery. • Lymphocyte viability. • Sterility. • Consistent density (1.077-1.080 at 20°C). Packaging • Packaged in amber, screw-cap bottles. • 5 x 100 ml bottles per carton. Storage. • Stored at room temperature. Reference Boyum, A. (1968): Isolation of mononuclear cells and granulocytes from human blood. Scand J. Clin. Lab. Invest. 21, Suppl. 97. Aspirate I IC[OI I IC~ LJI Catalog number: 8410-01 & discard serum Lymphocyte For Laboratory Use Aspirate layer & use (mononuclear Please write for our current Price List and Catalog. cells and Original platelets) ITi BIONETICS° LSM layer Erythrocytes Laboratory Products and Litton granulocytes 5516 Nicholson Lane, Kensington, Maryland 20795 Telephone: (301) 881-1557 1979 Litton Bionetics, tnc Get the most out of your high quality cytotoxic antibodies with LOW-TOX-M RABBIT COMPLEMENT LOW TOXICITY HIGH ACTIVITY Presentation: CL 3051 5 x 1 ml, lyophilized $30.00 When it comes to COMPLEMENT... come to CEDARLANE Direct orders or inquiries to: UNITED STATES: WORLDWIDE EXCEPT U.S. ,4 C~L CEDARLANE ACCURATE CHEMICAL & LABORATO RI ES SCIENTIFIC CORPORATION LIMITED 5516-8TH LINE, R.R. 2 28 TEC STREET, HICKSVtLLE, N.Y. 11801 HORNBY, ONTARIO, CANADA LOP 1E0 Telephone -

Stress and the Immune System Tracy B
4 World Health • 47th Yeor, No. 2, Morch-Aprill994 Stress and the immune system Tracy B. Herbert any people have the effects of factors as diverse as experienced the examinations, bereavement, divorce, Mconnection between stress unemployment, mental arithmetic, and getting sick. Colds, influenza, and looking after a relative with herpes and allergies seem worse Alzheimer's di sease. In general, when we are severely stressed at these studies find that stress is work or in the home. Others are related to changes in both the never sick until they go on vacation numbers of white blood cells in (that is, after the stress is over), and circulation and the quantity of then they spend the whole time antibody in the blood. Moreover, fighting the virus. Because of stress is associated with changes in intrinsic connections like these, the functioning of immune cells. many researchers are today That is, there is a relatively large exploring whether (and how) stress decrease in both lymphocyte and illness are actually linked. One proliferation and natural killer cell specific focus of this research is to activity in individuals who have study the effects of stress on the experienced stress. There seems to immune systems; after all, if stress A lymphocyte: stress may weaken the capacity be some connection between the affects immunity, that would be one of lymphocytes to combat infection. duration of the stress and the amount way in which stress could contribute of immune change. For example, the to illness. longer the stress, the greater the The function of the immune proliferation"- by incubating these decrease in the number of specific system is to protect us from cells for several days with types of white blood cells. -

Memory T Cells + Maintenance of CD8 the Dual Role of IL-2 in the Generation
The Dual Role of IL-2 in the Generation and Maintenance of CD8 + Memory T Cells Zhenhua Dai, Bogumila T. Konieczny and Fadi G. Lakkis This information is current as J Immunol 2000; 165:3031-3036; ; of September 30, 2021. doi: 10.4049/jimmunol.165.6.3031 http://www.jimmunol.org/content/165/6/3031 Downloaded from References This article cites 31 articles, 17 of which you can access for free at: http://www.jimmunol.org/content/165/6/3031.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on September 30, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2000 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Dual Role of IL-2 in the Generation and Maintenance of CD8؉ Memory T Cells1 Zhenhua Dai, Bogumila T. Konieczny, and Fadi G. Lakkis2 The mechanisms responsible for the generation and maintenance of T cell memory are unclear. In this study, we tested the role of IL-2 in allospecific CD8؉ T cell memory by analyzing the long-term survival, phenotype, and functional characteristics of IL-2-replete (IL-2؉/؉) and IL-2-deficient (IL-2؊/؊) CD8؉ TCR-transgenic lymphocytes in an adoptive transfer model. -

Lymphopenia-Induced Proliferation Lymphocytes During and After
The Journal of Immunology Effects of Increasing IL-7 Availability on Lymphocytes during and after Lymphopenia-Induced Proliferation1 Nabil Bosco,* Fabien Agene`s,* and Rhodri Ceredig2† IL-7 is critically involved in regulating peripheral T cell homeostasis. To investigate the role of IL-7 on lymphopenia-induced proliferation of polyclonal lymphocytes, we have transferred CFSE-labeled cells into a novel T-lymphopenic, IL-7-transgenic mouse line. Results obtained indicate that T and B cells do not respond in the same way to IL-7-homeostatic signals. Overex- pression of IL-7 enhances proliferation of both CD4؉ and CD8؉ T cells but with distinctly temporal effects. Expansion of naturally arising CD4؉-regulatory T cells was like that of conventional CD4؉ T cells. IL-7 had no effect on B cell proliferation. By immunohistology, transferred T cells homed to T cell areas of spleen lymphoid follicles. Increasing IL-7 availability enhanced T cell recovery by promoting cell proliferation and reducing apoptosis during early stages of lymphopenia-induced proliferation. Taken together, these results provide new insights into the pleiotropic effects of IL-7 on lymphopenia-induced T cell proliferation. The Journal of Immunology, 2005, 175: 162–170. espite declining thymic output with age, the peripheral T either IL-7-deficient or wild-type mice treated with anti-IL-7 or cell pool of an adult animal remains remarkably stable anti-IL-7R␣ mAb show that perturbation of IL-7 signals prevents D (1, 2). How the T cell pool is maintained remains a cen- the expansion of transferred T cells (1, 10). -

Association Between Neutrophil-Lymphocyte Ratio and Herpes Zoster Infection in 1688 Living Donor Liver Transplantation Recipients at a Large Single Center
biomedicines Article Association between Neutrophil-Lymphocyte Ratio and Herpes Zoster Infection in 1688 Living Donor Liver Transplantation Recipients at a Large Single Center Ji-Hoon Sim, Young-Jin Moon, Sung-Hoon Kim, Kyoung-Sun Kim , Ju-Seung Lee, Jun-Gol Song * and Gyu-Sam Hwang Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea; [email protected] (J.-H.S.); [email protected] (Y.-J.M.); [email protected] (S.-H.K.); [email protected] (K.-S.K.); [email protected] (J.-S.L.); [email protected] (G.-S.H.) * Correspondence: [email protected]; Tel.: +82-2-3010-3869 Abstract: Liver transplantation (LT) is closely associated with decreased immune function, a contrib- utor to herpes zoster (HZ). However, risk factors for HZ in living donor LT (LDLT) remain unknown. Neutrophil-lymphocyte ratio (NLR) and immune system function are reportedly correlated. This study investigated the association between NLR and HZ in 1688 patients who underwent LDLT between January 2010 and July 2020 and evaluated risk factors for HZ and postherpetic neuralgia (PHN). The predictive power of NLR was assessed through the concordance index and an integrated discrimination improvement (IDI) analysis. Of the total cohort, 138 (8.2%) had HZ. The incidence of HZ after LT was 11.2 per 1000 person-years and 0.1%, 1.3%, 2.9%, and 13.5% at 1, 3, 5, and 10 years, Citation: Sim, J.-H.; Moon, Y.-J.; Kim, respectively. In the Cox regression analysis, preoperative NLR was significantly associated with HZ S.-H.; Kim, K.-S.; Lee, J.-S.; Song, J.-G.; (adjusted hazard ratio [HR], 1.05; 95% confidence interval [CI], 1.02–1.09; p = 0.005) and PHN (HR, Hwang, G.-S.