The Progeria Syndrome Fact Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

De Barsy Syndrome: Orthopedic Case Report and Literature Review
MOJ Orthopedics & Rheumatology De Barsy Syndrome: Orthopedic Case report and Literature Review Introduction Case Report Volume 7 Issue 5 - 2017 This condition was first described in 1968 by De Barsy who and degeneration of the elastic tissue of the cornea and skin, Jose de Jesus Guerra Jasso1*, Douglas reported a case of a patient with progeria, dwarfism, oligofrenia and since then, it is known as Barsy Syndrome or Barsy- Colmenares Bonilla2 and Loreett Ocampo Perez3 of progeroid aspect, cutis laxa, corneal opacity, intrauterine 1Pediatric Orthopedics, Hospital Regional de Alta Especialidad growthMoens-Dierckx retardation Syndrome. and severe This ismental defined retardation as the combination (although del Bajio, Mexico some will learn to speak, intelligence is less than normal) [1]. 2Pediatric Orthopedic Service, Hospital regional de alta especialidad del bajio The orthopedic manifestations are dysplasia of hip development, 3Fellow of Pediatric Orthopedics, Hospital Regional de Alta hyper laxity of severe joints, athetoid movements, scoliosis and Especialidad del Bajio, Mexico severe deformities of the foot. Epidemiology in Latin America is unknown because of its underdiagnosis and when confused *Corresponding author: Jesus Guerra Jasso, Hospital with other connective tissue pathologies (Hutchinson-Gilford Regional de Alta Especialidad del Bajio, Boulevard Milenio No. 130. Col, San Carlos la Roncha, C.P. 37660, Guanajuato, syndrome, gerodermic osteodiplasia, even Ehlers-Danlos), the Mexico, Tel: 477-267 2000; Ext-1403; life expectancy of these patients varies according to the degree of Email: penetrance and in the world literature, there are very few reports (about 50), so the diagnosis requires a challenge [2]. Received: January 13, 2017 | Published: March 21, 2017 Clinical Case and thick clamp. -

DNA Damage in the Oligodendrocyte Lineage and Its Role in Brain Aging
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Mech Ageing Manuscript Author Dev. Author Manuscript Author manuscript; available in PMC 2018 January 01. Published in final edited form as: Mech Ageing Dev. 2017 January ; 161(Pt A): 37–50. doi:10.1016/j.mad.2016.05.006. DNA damage in the oligodendrocyte lineage and its role in brain aging Kai-Hei Tse1,2 and Karl Herrup1 1 Division of Life Science, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong Abstract Myelination is a recent evolutionary addition that significantly enhances the speed of transmission in the neural network. Even slight defects in myelin integrity impair performance and enhance the risk of neurological disorders. Indeed, myelin degeneration is an early and well-recognized neuropathology that is age associated, but appears before cognitive decline. Myelin is only formed by fully differentiated oligodendrocytes, but the entire oligodendrocyte lineage are clear targets of the altered chemistry of the aging brain. As in neurons, unrepaired DNA damage accumulates in the postmitotic oligodendrocyte genome during normal aging, and indeed may be one of the upstream causes of cellular aging - a fact well illustrated by myelin co-morbidity in premature aging syndromes arising from deficits in DNA repair enzymes. The clinical and experimental evidence from Alzheimer's disease, progeroid syndromes, ataxia-telangiectasia and other conditions strongly suggest that oligodendrocytes may in fact be uniquely vulnerable to oxidative DNA damage. If this damage remains unrepaired, as is increasingly true in the aging brain, myelin gene transcription and oligodendrocyte differentiation is impaired. Delineating the relationships between early myelin loss and DNA damage in brain aging will offer an additional dimension outside the neurocentric view of neurodegenerative disease. -

Atypical Progeroid Syndrome ID: 20-0188; April 2021 (P.E262K LMNA) DOI: 10.1530/EDM-20-0188
ID: 20-0188 -20-0188 M Yukina and others Atypical progeroid syndrome ID: 20-0188; April 2021 (p.E262K LMNA) DOI: 10.1530/EDM-20-0188 Atypical progeroid syndrome (p.E262K LMNA mutation): a rare cause of short stature and osteoporosis Correspondence Marina Yukina, Nurana Nuralieva, Ekaterina Sorkina, Ekaterina Troshina, should be addressed Anatoly Tiulpakov, Zhanna Belaya and Galina Melnichenko to E Sorkina Email Endocrinology Research Centre, Moscow, Russia [email protected] Summary Lamin A/C (LMNA) gene mutations cause a heterogeneous group of progeroid disorders, including Hutchinson–Gilford progeria syndrome, mandibuloacral dysplasia, atypical progeroid syndrome (APS) and generalized lipodystrophy- associated progeroid syndrome (GLPS). All of those syndromes are associated with some progeroid features, lipodystrophyandmetaboliccomplicationsbutvarydifferentlydependingonaparticularmutationandevenpatients carrying the same gene variant are known to have clinical heterogeneity. We report a new 30-year-old female patient from Russia with an APS and generalized lipodystrophy (GL) due to the heterozygous de novo LMNA p.E262K mutation and compare her clinical and metabolic features to those of other described patients with APS. Despite many health issues, short stature, skeletal problems, GL and late diagnosis of APS, our patient seems to be relatively metabolically healthy for her age when compared to previously described patients with APS. Learning points: • Atypicalprogeroidsyndromes(APS)arerareandheterogenicwithdifferentageofonsetanddegreeofmetabolic -
Neurodegeneration in Accelerated Aging
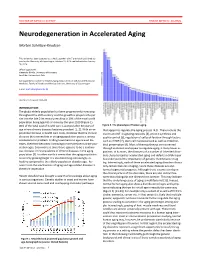
DOCTOR OF MEDICAL SCIENCE DANISH MEDICAL JOURNAL Neurodegeneration in Accelerated Aging Morten Scheibye-Knudsen This review has been accepted as a thesis together with 7 previously published pa- pers by the University of Copenhagen, October 16, 2014 and defended on January 14, 2016 Official opponents: Alexander Bürkle, University of Konstanz Lars Eide, University of Oslo Correspondence: Center for Healthy Aging, Department of Cellular and Molecular Medicine, Faculty of Health and Medical Sciences, University of Copenhagen E-mail: [email protected] Dan Med J 2016;63(11):B5308 INTRODUCTION The global elderly population has been progressively increasing throughout the 20th century and this growth is projected to per- sist into the late 21st century resulting in 20% of the total world population being aged 65 or more by the year 2100 (Figure 1). 80% of the total cost of health care is accrued after 40 years of Figure 2. The phenotype of human aging. age where chronic diseases become prevalent [1, 2]. With an ex- that appear to regulate the aging process [4,5]. These include the ponential increase in health care costs, it follows that the chronic insulin and IGF-1 signaling cascades [4], protein synthesis and diseases that accumulate in an aging population poses a serious quality control [6], regulation of cell proliferation through factors socioeconomic problem. Finding treatments to age related dis- such as mTOR [7], stem cell maintenance 8 as well as mitochon- eases, therefore becomes increasingly more pertinent as the pop- drial preservation [9]. Most of these pathways are conserved ulation ages. Even more so since there appears to be a continu- through evolution and appear to regulate aging in many lower or- ous increase in the prevalence of chronic diseases in the aging ganisms. -

Trichothiodystrophy
Trichothiodystrophy Author: Doctor Alfredo Rossi1 and Doctor C. Cantisani. Creation date: June 2004 Scientific Editor: Prof Antonella Tosti 1Dipartimento di Malattie Cutanee-Veneree Chirurgia Plastica-Ricostruttiva, Università degli studi di Roma “La Sapienza” Abstract Keywords Definition Epidemiology Etiology Clinical description Diagnostic methods Prenatal diagnosis Management References Abstract Trichothiodystrophy (TTD) is a rare autosomal recessive genetic disorder characterized by abnormal synthesis of the sulphur containing keratins and consequently hair dysplasia, associated with numerous symptoms affecting mainly organs derived from the neuroectoderm. This phenotypic aspect is due to mutations in the DNA-dependent ATPase/helicase subunit of TFIIH, XPB and XPD. Abnormalities in excision repair of ultraviolet (UV)-damaged DNA are recognized in about half of the patients. The clinical appearance is characterized by brittle and fragile hair, congenital ichthyosis, nail and dental dysplasias, cataract, progeria-like face, growth and mental retardation. The abnormalities are usually obvious at birth, with variable clinical expression. The variants of TTD, depending on their different associations, are known by the initials BIDS, IBIDS, PIBIDS, SIBIDS, ONMRS, as well as the eponyms of the Pollit, Tay, Sabinas syndromes or Amish brittle hair. The exact prevalence of TTD is unknown, but appears to be rather uncommon. About 20 cases of PIBI(D)S have been reported in the literature. Up to 1991, clinical data of 15 cases with IBIDS were published. Prenatal diagnostic of TTD is available. There is no specific treatment. Keywords Brittle hair, photosensitivity, ichthyosis, BIDS, IBIDS, PIBIDS, SIBIDS, ONMRS, Tay-syndrome Definition tail pattern). They named it Trichothiodystrophy, Trichothiodystrophy (TTD) is a group of rare noticing also an increased Photosensitivity and autosomal recessive disorders with heterogenic Ichthyosis in these patients (PIBIDS). -

Molecular Basis of Progeroid Syndromes–S–S– the Wwthe Erner Andanderner Hutchinson-Gilford Syndromes
Proc. Indian natn Sci Acad. B69 No. 4 pp 625-640 (2003) Molecular Basis of Progeroid Syndromes–s–s– the WWthe erner andanderner Hutchinson-Gilford Syndromes JUNKO OSHIMA*, NANCY B HANSON and GEORGE M MARTIN Department of Pathology, University of W ashington, Seattle, WA 98195, USA (Received on 17 July 2003; Accepted after r evision on 6 August 2003) Segmental progeroid syndromes are members of a group of disorders in which affected individuals present various featur es suggestive of accelerated aging. The two best-known examples are the Werner syndro m e (WS; “Progeria of the adult”) and the Hutchinson-Gilford Progeria syndrome (HGPS; “Progeria of child- hood”). The gene responsible for WS, WRN, was identified in 1996 and encodes a multifunctional nuclear protein with exonuclease and helicase domains. WS patients and cells isolated from the WS patients show various genomic instability phenotypes, including an incr eased incidence of cancer. The WRN protein is thought to play a crucial role in optimizing the regulation of DNA repair processes. Recently, a novel r ecurr ent mutation in the LMNA gene has been shown to be responsible for HGPS. LMNA encodes nuclear intermediate filaments, lamins A and C; mutant lamins are thought to result in nuclear fragility. Ther e ar e at least six other disor ders caused by LMNA mutations, most of which affect cells and tissues of mesenchymal origins, including atypical forms of WS. The pathophysiologies of these and certain other progeroid syndromes indicate an important role for DNA damage in the genesis of common age- related disorders. Key WWKey ords: WWords: erner syndrome, WRN, RecQ, Hutchinson-Gilford Progeria syndrome, LMNA, Lamin, Genomic instability, Aging, Human Introduction of WS, previously based upon clinical criteria, can Segmental progeroid syndromes encompass a now be confirmed by molecular biological methods. -

Download PDF (1878K)
2018, 65 (2), 227-238 Note Definitive diagnosis of mandibular hypoplasia, deafness, progeroid features and lipodystrophy (MDPL) syndrome caused by a recurrent de novo mutation in the POLD1 gene Haruka Sasaki1), 2), Kumiko Yanagi3) *, Satoshi Ugi4), Kunihisa Kobayashi1), Kumiko Ohkubo5), Yuji Tajiri6), Hiroshi Maegawa4), Atsunori Kashiwagi7) and Tadashi Kaname3) * 1) Department of Endocrinology and Diabetes Mellitus, Fukuoka University Chikushi Hospital, Chikushino, Fukuoka 818-8502, Japan 2) Division of Diabetic Medicine, Bunyukai Hara Hospital, Ohnojo, Fukuoka 816-0943, Japan 3) Department of Genome Medicine, National Research Institute for Child Health, Setagaya, Tokyo 157-8535, Japan 4) Department of Medicine, Shiga University of Medical Science, Otsu, Shiga 520-2192, Japan 5) Department of Laboratory Medicine, School of Medicine, Fukuoka University, Jonan-ku, Fukuoka 814-0180, Japan 6) Division of Endocrinology and Metabolism, Kurume University School of Medicine, Kurume, Fukuoka 830-0111, Japan 7) Diabetes Center, Seikokai Kusatsu General Hospital, Kusatsu, Shiga 525-8585, Japan Abstract. Segmental progeroid syndromes with lipodystrophy are extremely rare, heterogeneous, and complex multi-system disorders that are characterized by phenotypic features of premature aging affecting various tissues and organs. In this study, we present a “sporadic/isolated” Japanese woman who was ultimately diagnosed with mandibular hypoplasia, deafness, progeroid features, and progressive lipodystrophy (MDPL) syndrome (MIM #615381) using whole exome sequencing analysis. She had been suspected as having atypical Werner syndrome and/or progeroid syndrome based on observations spanning a 30-year period; however, repeated genetic testing by Sanger sequencing did not identify any causative mutation related to various subtypes of congenital partial lipodystrophy (CPLD) and/or mandibular dysplasia with lipodystrophy (MAD). -

1. Progeria 101: Frequently Asked Questions
1. PROGERIA 101: FREQUENTLY ASKED QUESTIONS 1. Progeria 101: Frequently Asked Questions What is Hutchinson-Gilford Progeria Syndrome? What is PRF’s history and mission? What causes Progeria? How is Progeria diagnosed? Are there different types of Progeria? Is Progeria contagious or inherited? What is Hutchinson-Gilford Progeria Syndrome (HGPS or Progeria)? Progeria is also known as Hutchinson-Gilford Progeria Syndrome (HGPS). Genetic testing for It was first described in 1886 by Dr. Jonathan Hutchinson and in 1897 by Progeria can be Dr. Hastings Gilford. Progeria is a rare, fatal, “premature aging” syndrome. It’s called a syndrome performed from a because all the children have very similar symptoms that “go together”. The small sample of blood children have a remarkably similar appearance, even though Progeria affects children of all different ethnic backgrounds. Although most babies with (1-2 tsp) or sometimes Progeria are born looking healthy, they begin to display many characteristics from a sample of saliva. of accelerated aging by 18-24 months of age, or even earlier. Progeria signs include growth failure, loss of body fat and hair, skin changes, stiffness of joints, hip dislocation, generalized atherosclerosis, cardiovascular (heart) disease, and stroke. Children with Progeria die of atherosclerosis (heart disease) or stroke at an average age of 13 years (with a range of about 8-21 years). Remarkably, the intellect of children with Progeria is unaffected, and despite the physical changes in their young bodies, these extraordinary children are intelligent, courageous, and full of life. 1.2 THE PROGERIA HANDBOOK What is PRF’s history and mission? The Progeria Research Foundation (PRF) was established in the United States in 1999 by the parents of a child with Progeria, Drs. -

Human Radiosensitivity and Radiosusceptibility: What Are the Differences?
International Journal of Molecular Sciences Review Human Radiosensitivity and Radiosusceptibility: What Are the Differences? Laura El-Nachef 1, Joelle Al-Choboq 1, Juliette Restier-Verlet 1, Adeline Granzotto 1, Elise Berthel 1,2, Laurène Sonzogni 1,Mélanie L. Ferlazzo 1, Audrey Bouchet 1 , Pierre Leblond 3, Patrick Combemale 3, Stéphane Pinson 4, Michel Bourguignon 1,5 and Nicolas Foray 1,* 1 Inserm, U1296 unit, Radiation: Defense, Health and Environment, Centre Léon-Bérard, 28, rue Laennec, 69008 Lyon, France; [email protected] (L.E.-N.); [email protected] (J.A.-C.); Juliette.Restier–[email protected] (J.R.-V.); [email protected] (A.G.); [email protected] (E.B.); [email protected] (L.S.); [email protected] (M.L.F.); [email protected] (A.B.); [email protected] (M.B.) 2 Neolys Diagnostics, 67960 Entzheim, France 3 Centre Léon-Bérard, 28, rue Laennec, 69008 Lyon, France; [email protected] (P.L.); [email protected] (P.C.) 4 Hospices Civils de Lyon, Quai des Célestins, 69002 Lyon, France; [email protected] 5 Université Paris Saclay Versailles St Quentin en Yvelines, 78035 Versailles, France * Correspondence: [email protected]; Tel.: +33-4-78-78-28-28 Abstract: The individual response to ionizing radiation (IR) raises a number of medical, scientific, and societal issues. While the term “radiosensitivity” was used by the pioneers at the beginning Citation: El-Nachef, L.; Al-Choboq, of the 20st century to describe only the radiation-induced adverse tissue reactions related to cell J.; Restier-Verlet, J.; Granzotto, A.; death, a confusion emerged in the literature from the 1930s, as “radiosensitivity” was indifferently Berthel, E.; Sonzogni, L.; Ferlazzo, used to describe the toxic, cancerous, or aging effect of IR. -

Early Onset Diabetes in Two Children Due to Progeria, a Monogenic Disease of DNA Repair
CASE REPORT DO I: 10.4274/jcrpe.galenos.2019.2019.0126 J Clin Res Pediatr Endocrinol 2020;12(3):315-318 Early Onset Diabetes in Two Children due to Progeria, a Monogenic Disease of DNA Repair Martin Holder¹, Valerie Schwitzgebel² ¹Klinikum Stuttgart, Olgahospital, Department of Pediatric Endocrinology and Diabetology, Stuttgart, Germany ²Hopital des Enfants, Endocrinologie et Diabetologie Pediatriques, Geneve, Switzerland What is already known on this topic? Less is known about type 2 like diabetes mellitus in children and adolescents with progeria-syndrome although they have a high risk of developing diabetes mellitus. What this study adds? Early and regular screening for diabetes mellitus are mandatory. Treatment with metformin at an early stage should be recommended to prevent early symptoms of diabetes and potentially delay the clinical course of progeria Abstract Progeria syndrome is a rare disorder in childhood which causes accelerated systemic aging. Due to the accelerated aging process, disorders which normally occur only in old age will appear in these children at a much younger age. We report two children with progeria syndrome, in whom fulminant diabetes mellitus manifested at a very early age. Keywords: Progeria syndrome, diabetes mellitus, metformin, prevention. Introduction cardiovascular disease can occur. Additionally, they may have audiologic, dental, and ophthalmologic issues Progeria syndrome is a group of very rare genetic disorders that impair their lives. Less is known about metabolic which are characterized by premature aging and classified complications in children with progeria syndrome. In by various names based on causative etiology: Hutchinson- WS, also known as adult progeroid syndrome, type 2 like Gilford progeria syndrome (HGPS), Néstor-Guillermo progeria diabetes mellitus is one of the clinical manifestations of syndrome, atypical progeria syndromes, restrictive dermopathy, the disease and attention must give to the differential mandibuloacral dysplasia, Werner syndrome (WS), Bloom diagnosis (1). -

Werner and Hutchinson–Gilford Progeria Syndromes: Mechanistic Basis of Human Progeroid Diseases
REVIEWS MECHANISMS OF DISEASE Werner and Hutchinson–Gilford progeria syndromes: mechanistic basis of human progeroid diseases Brian A. Kudlow*¶, Brian K. Kennedy* and Raymond J. Monnat Jr‡§ Abstract | Progeroid syndromes have been the focus of intense research in part because they might provide a window into the pathology of normal ageing. Werner syndrome and Hutchinson–Gilford progeria syndrome are two of the best characterized human progeroid diseases. Mutated genes that are associated with these syndromes have been identified, mouse models of disease have been developed, and molecular studies have implicated decreased cell proliferation and altered DNA-damage responses as common causal mechanisms in the pathogenesis of both diseases. Progeroid syndromes are heritable human disorders with therefore termed segmental, as opposed to global, features that suggest premature ageing1. These syndromes progeroid syndromes. Among the segmental progeroid have been well characterized as clinical disease entities, syndromes, the syndromes that most closely recapitu- and in many instances the associated genes and causative late the features of human ageing are Werner syndrome mutations have been identified. The identification of (WS), Hutchinson–Gilford progeria syndrome (HGPS), genes that are associated with premature-ageing-like Cockayne syndrome, ataxia-telangiectasia, and the con- syndromes has increased our understanding of molecu- stitutional chromosomal disorders of Down, Klinefelter lar pathways that protect cell viability and function, and -

DNA Replication Timing Alterations Identify Common Markers Between Distinct Progeroid Diseases
DNA replication timing alterations identify common PNAS PLUS markers between distinct progeroid diseases Juan Carlos Rivera-Muliaa, Romain Despratb, Claudia Trevilla-Garciaa, Daniela Cornacchiac, Hélène Schwererd, Takayo Sasakia, Jiao Simaa, Tyler Fellsa, Lorenz Studerc, Jean-Marc Lemaitreb,d,1, and David M. Gilberta,e,1 aDepartment of Biological Science, Florida State University, Tallahassee, FL 32306; bPlateforme de Reprogrammation Cellulaire (SAFE-iPSC) Stem Cell Core Facility, Infrastructure Nationale d’Ingénierie des Cellules Souches Pluripotentes et Cellules Différenciées (INGESTEM), Centres Hospitaliers Universitaires de Montpellier, Hopital Saint Eloi, F3400 Montpellier, France; cThe Center for Stem Cell Biology, Memorial Sloan-Kettering Institute, New York, NY 10065; dInstitute of Regenerative Medicine and Biotherapies, U1183 INSERM, Université de Montpellier, F3400 Montpellier, France; and eCenter for Genomics and Personalized Medicine, Florida State University, Tallahassee, FL 32306 Edited by Terry L. Orr-Weaver, Whitehead Institute, Cambridge, MA, and approved November 8, 2017 (received for review June 30, 2017) Progeroid syndromes are rare genetic disorders that phenotypi- the mechanisms linking the cellular defects to pathophysiological cally resemble natural aging. Different causal mutations have been manifestations of the disease. identified, but no molecular alterations have been identified that Previously, we demonstrated that the temporal order of ge- are in common to these diseases. DNA replication timing (RT) is a