Thalamic Stimulation Improves Postictal Cortical Arousal and Behavior
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

EPILEPSY and OTHER SEIZURE DISORDERS 273 Base of Rh
45077 Ropper: Adams and Victor’s Principles of Neurology, 8/E McGraw-Hill BATCH RIGHT top of rh CHAPTER 16 base of rh EPILEPSY AND OTHER cap height base of text SEIZURE DISORDERS In contemporary society, the frequency and importance of epilepsy logic disease that demands the employment of special diagnostic can hardly be overstated. From the epidemiologic studies of Hauser and therapeutic measures, as in the case of a brain tumor. and colleagues, one may extrapolate an incidence of approximately A more common and less grave circumstance is for a seizure 2 million individuals in the United States who are subject to epi- to be but one in an extensive series recurring over a long period of lepsy (i.e., chronically recurrent cerebral cortical seizures) and pre- time, with most of the attacks being more or less similar in type. dict about 44 new cases per 100,000 population occur each year. In this instance they may be the result of a burned-out lesion that These figures are exclusive of patients in whom convulsions com- originated in the past and remains as a scar. The original disease plicate febrile and other intercurrent illnesses or injuries. It has also may have passed unnoticed, or perhaps had occurred in utero, at been estimated that slightly less than 1 percent of persons in the birth, or in infancy, in parts of the brain inaccessible for exami- United States will have epilepsy by the age of 20 years (Hauser nation or too immature to manifest signs. It may have affected a and Annegers). -
Handbook of EEG INTERPRETATION This Page Intentionally Left Blank Handbook of EEG INTERPRETATION
Handbook of EEG INTERPRETATION This page intentionally left blank Handbook of EEG INTERPRETATION William O. Tatum, IV, DO Section Chief, Department of Neurology, Tampa General Hospital Clinical Professor, Department of Neurology, University of South Florida Tampa, Florida Aatif M. Husain, MD Associate Professor, Department of Medicine (Neurology), Duke University Medical Center Director, Neurodiagnostic Center, Veterans Affairs Medical Center Durham, North Carolina Selim R. Benbadis, MD Director, Comprehensive Epilepsy Program, Tampa General Hospital Professor, Departments of Neurology and Neurosurgery, University of South Florida Tampa, Florida Peter W. Kaplan, MB, FRCP Director, Epilepsy and EEG, Johns Hopkins Bayview Medical Center Professor, Department of Neurology, Johns Hopkins University School of Medicine Baltimore, Maryland Acquisitions Editor: R. Craig Percy Developmental Editor: Richard Johnson Cover Designer: Steve Pisano Indexer: Joann Woy Compositor: Patricia Wallenburg Printer: Victor Graphics Visit our website at www.demosmedpub.com © 2008 Demos Medical Publishing, LLC. All rights reserved. This book is pro- tected by copyright. No part of it may be reproduced, stored in a retrieval sys- tem, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of the publisher. Library of Congress Cataloging-in-Publication Data Handbook of EEG interpretation / William O. Tatum IV ... [et al.]. p. ; cm. Includes bibliographical references and index. ISBN-13: 978-1-933864-11-2 (pbk. : alk. paper) ISBN-10: 1-933864-11-7 (pbk. : alk. paper) 1. Electroencephalography—Handbooks, manuals, etc. I. Tatum, William O. [DNLM: 1. Electroencephalography—methods—Handbooks. WL 39 H23657 2007] RC386.6.E43H36 2007 616.8'047547—dc22 2007022376 Medicine is an ever-changing science undergoing continual development. -

Epilepsy and Psychosis
Central Journal of Neurological Disorders & Stroke Review Article Special Issue on Epilepsy and Psychosis Epilepsy and Seizures *Corresponding author Daniel S Weisholtz* and Barbara A Dworetzky Destînâ Yalcin A, Department of Neurology, Ümraniye Department of Neurology, Brigham and Women’s Hospital, Harvard University, Boston Research and Training Hospital, Istanbul, Turkey, Massachusetts, USA Email: Submitted: 11 March 2014 Abstract Accepted: 08 April 2014 Psychosis is a significant comorbidity for a subset of patients with epilepsy, and Published: 14 April 2014 may appear in various contexts. Psychosis may be chronic or episodic. Chronic Interictal Copyright Psychosis (CIP) occurs in 2-10% of patients with epilepsy. CIP has been associated © 2014 Yalcin et al. most strongly with temporal lobe epilepsy. Episodic psychoses in epilepsy may be classified by their temporal relationship to seizures. Ictal psychosis refers to psychosis OPEN ACCESS that occurs as a symptom of seizure activity, and can be seen in some cases of non- convulsive status epilepticus. The nature of the psychotic symptoms generally depends Keywords on the localization of the seizure activity. Postictal Psychosis (PIP) may occur after • Epilepsy a cluster of complex partial or generalized seizures, and typically appears after • Psychosis a lucid interval of up to 72 hours following the immediate postictal state. Interictal • Hallucinations psychotic episodes (in which there is no definite temporal relationship with seizures) • Non-convulsive status epilepticus may be precipitated by the use of certain anticonvulsant drugs, particularly vigabatrin, • Forced normalization zonisamide, topiramate, and levetiracetam, and is linked in some cases to “forced normalization” of the EEG or cessation of seizures, a phenomenon known as alternate psychosis. -

Epilepsy E1 (1)
EPILEPSY E1 (1) Epilepsy Last updated: September 9, 2021 CLASSIFICATION ............................................................................................................................................ 3 FOUR‐DIMENSIONAL EPILEPSY CLASSIFICATION (LÜDERS 2019).................................................................. 3 Semiology ............................................................................................................................................... 3 Epileptogenic zone ................................................................................................................................. 4 Etiology .................................................................................................................................................. 5 ILAE (2017) ................................................................................................................................................ 5 SEMIOLOGY (CLINICAL MANIFESTATIONS) - DEFINITIONS ......................................................................... 5 SEIZURE TRIGGERING FACTORS .................................................................................................................... 5 MOTOR ........................................................................................................................................................ 6 Elementary Motor .................................................................................................................................. 6 Automatism ........................................................................................................................................... -

Pediatric Seizures Disclosures Learning Objectives Definitions
1/15/2020 Disclosures Relevant Financial Relationship(s) None Pediatric Seizures Off Label Usage James Miles, MD None NDAFP Family Medicine Update January 22, 2020 1 2 Learning Objectives Definitions • Review the key concepts of pediatric seizures. • Seizure : clinical expression of abnormal, • Appreciate how to evaluate a child with spells excessive, synchronous discharges of neurons concerning for seizure. residing primarily in the cerebral cortex. • Highlight the different types of treatment of • Epilepsy : at least two unprovoked seizures pediatric seizures. occurring more than 24 hours apart. • Be able to identify some common pediatric • Provoked seizure : secondary cause such as seizure disorders. hyponatremia, hypocalcemia, high fever, toxic • Differentiate between seizures and nonepileptic exposure, intracranial bleeding, or bacterial spells. meningitis. 3 4 Epidemiology Seizure Semiology • 0.5-1% kids will experience at least one • Generalized seizures afebrile seizure by adolescence – Tonic/Tonic-Clonic • 3-5% kids at least one febrile seizure – Absence – 3-6% will develop epilepsy – Myoclonic • 3.6% experiencing at least one seizure in an • Focal seizures 80-year lifespan – Tonic/Tonic-Clonic • Risk of having a seizure is greatest in infancy – Temporal lobe and after age 60 – Secondary generalization • M>F, higher in lower socioeconomic groups 5 6 1 1/15/2020 Pediatric Etiology Adult Etiology • Genetic • Ischemic or hemorrhagic stroke • Structural: injury, neurodevelopment • Traumatic head injury and bleeds • Metabolic: fever, -

MRI Reveals New Epilepsy Surgery Candidates
14 Epilepsy C LINICAL N EUROLOGY N EWS • February 2007 MRI Reveals New Epilepsy Surgery Candidates BY MICHELE G. SULLIVAN spherectomy a difficult Mid-Atlantic Bureau decision for both physi- cian and parent. emispherectomy or cortical resection may cure At a recent meeting of catastrophic epilepsy even in a child with gener- the Child Neurology So- alized seizures, with MRI rather than EEG iden- ciety, Dr. Wyllie reported H YLLIE tifying surgical candidates, according to Dr. Elaine Wyl- some preliminary find- W lie, director of the child neurology center at the Cleveland ings from a retrospective Clinic Children’s Hospital. study of 50 such children, LAINE . E R Candidates for the surgery are children in whom the all of whom underwent D epileptogenic focus is a unilateral brain lesion that prob- functional hemispherec- ably occurred prenatally, perinatally, or during infancy. tomy or cortical resection “These extensive congenital or early-acquired unilateral at the Cleveland Clinic in brain lesions may produce generalized—or even con- 1999-2004. All had ictal or HOTOS COURTESY tralateral—maximum epileptiform discharge patterns on interictal epileptiform dis- P EEG,” she said in an interview. “Despite these EEG find- charges that were either Encephalomalacia from a perinatal infarction is shown on MRI (left); perioperative ings, you can still get favorable surgical results in some generalized or maximum interictal EEG in the same patient shows generalized slow spike-wave complexes (right). cases, if you have a clear unilateral brain lesion on MRI contralateral to the surgi- with side-appropriate semiology.” Children with this cal site, and early brain lesions that were unilateral or very ated from high school, and is now learning a trade.” clinical picture are typically turned away from surgery. -

(CBT) in Psychogenic Non-Epileptic Seizures (PNES): a Case Report and Literature Review
behavioral sciences Article Cognitive Behavioral Therapy (CBT) in Psychogenic Non-Epileptic Seizures (PNES): A Case Report and Literature Review Saher Hoda Kamil 1, Mustafa Qureshi 2 and Rikinkumar S. Patel 3,* 1 Department of Psychiatry, Austin State Hospital, Austin, TX 78751, USA; [email protected] 2 Department of Psychiatry, Texas Tech University Health Science Center, Midland, TX 79701, USA; [email protected] 3 Department of Psychiatry, Griffin Memorial Hospital, Norman, OK 73071, USA * Correspondence: [email protected]; Tel.: +1-405-573-2199 Received: 28 December 2018; Accepted: 24 January 2019; Published: 29 January 2019 Abstract: Psychogenic non-epileptic seizures (PNES) are classified as a somatoform conversion disorder. We present a case of a 24-year-old male with a past psychiatric history of posttraumatic stress disorder (PTSD) and anxiety disorder, admitted to our inpatient psychiatric unit. The patient experienced multiple episodes of seizures during hospitalization. Work up was unremarkable, and PNES were suspected and later confirmed with video-electroencephalography (video-EEG). He underwent supervised withdrawal of antiepileptic medications with the initiation of cognitive behavioral therapy (CBT), which reduced the frequency of seizures. Diagnosis of PNES can present as a challenge and failure to diagnose its psychological nature can lead to a delay in the psychological intervention. CBT leads to a decrease in seizure frequency, and improvement in psychiatric symptoms, psychosocial functioning, and quality of life. It is important to consider PNES in the differential diagnosis of seizures presented by psychiatric patients, as CBT is necessary for better patient outcomes. Keywords: PNES; psychogenic non-epileptic seizures; pseudoseizures; CBT; cognitive behavioral therapy; management; inpatient psychiatry; EEG 1. -

Postictal Switch in Blood Flow Distribution and Temporal Lobe Seizures 893
Journal of Neurology, Neurosurgery, and Psychiatry 1992;55:891-894 891 Postictal switch in blood flow distribution and J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.55.10.891 on 1 October 1992. Downloaded from temporal lobe seizures Mark R Newton, Samuel F Berkovic, Mark C Austin, Christopher C Rowe, W John McKay, Peter F Bladin Abstract ictal studies with PET can be performed only The ictal increase of regional cerebral serendipitously when seizures happen to occur blood flow has yet to be fully utilised in the during the scan. SPECT radionuclides such investigation of focal seizures. Although as o9mTechnetium-hexamethyl propyleneami- single photon emission tomography neoxime (HMPAO) rapidly cross the blood- (SPECT) is being increasingly used in the brain barrier and reside in cerebral tissue for localisation of epileptic foci, the evolution several hours. Isotope can thus be injected and time courses of the peri-ictal perfu- during an observed seizure and the patient can sion changes have yet to be clarified. We then be scanned within the next few hours, performed serial SPECT studies in the with the scan reflecting cerebral blood flow interictal, ictal and immediate postictal (CBF) at the time ofinjection."4These proper- states in 12 patients with refractory tem- ties permit the prospective and serial evalu- poral lobe epilepsy to define the patterns ation of changes in CBF associated with focal and duration of peri-ictal cerebral blood seizures, which typically last less than two flow changes. Visual analysis showed a minutes. constant pattern of unilateral global We previously reported the early postictal increases in temporal lobe perfusion dur- perfusion changes with SPECT, which allowed ing seizures which suddenly switched to a accurate prediction of seizure focus in 69% of pattern of relative mesial temporal (hip- a series of patients with refractory temporal pocampal) hyperperfusion and lateral lobe epilepsy."-3 We have since sought to temporal hypoperfusion in the immediate determine the diagnostic value of ictal and postictal period. -

Status Epilepticus in Dogs and Cats, Part 1: Etiopathogenesis, Epidemiology, and Diagnosis
Clinical Practice Review Journal of Veterinary Emergency and Critical Care 27(3) 2017, pp 278–287 doi: 10.1111/vec.12605 Status epilepticus in dogs and cats, part 1: etiopathogenesis, epidemiology, and diagnosis Susan Blades Golubovic, DVM and John H. Rossmeisl Jr., DVM, MS, DACVIM Abstract Objective – To review current knowledge of the etiopathogenesis, diagnosis, and consequences of status epilep- ticus (SE) in veterinary patients. Data Sources – Human and veterinary literature, including clinical and laboratory research and reviews. Etiopathogenesis – Status epilepticus is a common emergency in dogs and cats, and may be the first manifes- tation of a seizure disorder. It results from the failure of termination of an isolated seizure. Multiple factors are involved in SE, including initiation and maintenance of neuronal excitability, neuronal network synchroniza- tion, and brain microenvironmental contributions to ictogenesis. Underlying etiologies of epilepsy and SE in dogs and cats are generally classified as genetic (idiopathic), structural-metabolic, or unknown. Diagnosis – Diagnosis of convulsive SE is usually made based on historical information and the nature of the seizures. Patient specific variables, such as the history, age of seizure onset, and physical and interictal neuro- logical examination findings can help hone the rule out list, and are used to guide selection and prioritization of diagnostic tests. Electroencephalographic monitoring is routinely used in people to diagnose SE and guide patient care decisions, but is infrequently performed in veterinary medicine. Nonconvulsive status epilepticus has been recognized in veterinary patients; routine electroencephalography would aid in the diagnosis of this phenomenon in dogs and cats. Clinical Sequelae – Status epilepticus is a medical emergency that can result in life-threatening complications involving the brain and systemic organs. -

Epilepsy Resource Manual
Public Awareness “Campaign-in-a-Box” Toolkit “Commitment To Excellence” Table of Contents 1 About West Virginia University Pediatric Neurology ...........1 2 What Is Epilepsy ...................................................................7 Types of Seizures ..................................................................8 Diagnosis ............................................................................12 Treatment ...........................................................................13 3 First Aid ..............................................................................17 4 Your Child at Home ............................................................19 5 Your Child at School ...........................................................23 6 Physical Fitness and Exercise .............................................35 7 Driving With Epilepsy .........................................................37 8 Helpful Forms .....................................................................39 9 Resources............................................................................47 10 Glossary .............................................................................63 This publication was made possible by a grant from the federal Health Resources and Services Administration (U23MC03909-03-01), and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the HRSA. 3 About West Virginia University Pediatric Neurology 1 West Virginia University Department of Pediatrics -

Gaze Palsy As a Manifestation of Todd's Phenomenon
brain sciences Case Report Gaze Palsy as a Manifestation of Todd’s Phenomenon: Case Report and Review of the Literature Karmele Olaciregui Dague 1,* , Manuel Dafotakis 2, Jörg B. Schulz 2,3 and Rainer Surges 1 1 Epileptology Center, Medical Faculty, University Hospital Bonn, Venusberg-Campus 1, 53127 Bonn, Germany; [email protected] 2 Department of Neurology, University Hospital RWTH Aachen, Pauwelsstraße 30, 52074 Aachen, Germany; [email protected] (M.D.); [email protected] (J.B.S.) 3 JARA-BRAIN Institute Molecular Neuroscience and Neuroimaging, Forschungszentrum Jülich GmbH and RWTH Aachen University, 52074 Aachen, Germany * Correspondence: [email protected] Received: 23 April 2020; Accepted: 13 May 2020; Published: 15 May 2020 Abstract: Background: Though Todd’s phenomenon (TP) is a relatively rare occurrence, its correct identification is of key diagnostic and therapeutic importance as a stroke mimic. Here we describe a case of isolated gaze palsy as a manifestation of TP, discuss periictal gaze abnormalities as lateralizing sign involving the frontal eye field (FEF), and present a narrative literature review. Methods: We reviewed the main features of the case and conducted a structured literature search of TP and gaze palsy using PubMed. We restricted the search to publications in English, Spanish, French, and German. Case presentation: A 71-year-old male with a history of right frontotemporal subarachnoid hemorrhage was admitted to the Emergency Department of our institution after suffering a first unprovoked focal to bilateral tonic-clonic seizure with ictal gaze deviation to the left. Cranial imaging showed no signs of ischemia, intracerebral hemorrhage, or tumor. The patient presented the following postictal features: involuntary eye deviation to the right due to left-sided gaze palsy and disorientation in time with preserved responsiveness. -
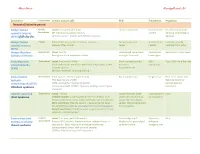
Ahmed Koriesh Neurologyresidents.Net
Ahmed Koriesh NeurologyResidents.Net Syndrome Classification Clinical picture (AE) EEG Treatment Prognosis Neonatal/Infantile period Benign familial Partial/ Onset: First week after birth. focal or multifocal No treatment Usually stop by 6 wks. neonatal seizures Generalized CP: Clonic or myoclonic seizures needed 16% risk of developing (BFNS) fifth day fits (AD inheritance - KCNQ2 and KCNQ3 mutation) epilepsy. Benign familial Partial Focal clonic seizure, Eye deviation, cyanosis Occipital-parietal No treatment Excellent, usually infantile seizures Seizures often cluster spikes needed resolves in 1-2 years (BFIS) Benign Myoclonic Generalized Onset: 6m:3y Generalized spike/wave Valproate & Remission in most cases Epilepsy of Infancy Brief generalized myoclonic activity lasting 2-3 seconds Lamotrigine Early Myoclonic Generalized Onset: first month of life Burst-suppression that AED - Poor, 50% die in few wks encephalopathy Starts with erratic myoclonic jerks then simple focal sz then evolve to Intractable (EME) infantile spasms. hypsarrhythmia. Multiple metabolic causes identified. Early infantile Generalized Tonic spasms - Often hundreds daily Burst-suppression Surgical eval. Poor - Can evolve into Epileptic 75% have lesions in MRI West syndrome or encephalopathy (EIEE) (Often arising from cortical dysplasia Lennox-Gastaut Ohtahara syndrome or associated with STXBP1 “Syntaxin binding protein” gene syndrome mutation) Infantile spasms & Generalized Onset: Infancy Hypsarrhythmia (high- Corticotrophin Poor West Syndrome Infantile spasms: sudden