COASTAL and GROUND WATER POLLUTION in KAVARATTI ISLAND, LAKSHADWEEP SEA Thesis Submitted to the COCHIN UNIVERSITY of SCIENCE 8: TECHNOLOGY
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sl No Localbody Name Ward No Door No Sub No Resident Name Address Mobile No Type of Damage Unique Number Status Rejection Remarks
Flood 2019 - Vythiri Taluk Sl No Localbody Name Ward No Door No Sub No Resident Name Address Mobile No Type of Damage Unique Number Status Rejection Remarks 1 Kalpetta Municipality 1 0 kamala neduelam 8157916492 No damage 31219021600235 Approved(Disbursement) RATION CARD DETAILS NOT AVAILABLE 2 Kalpetta Municipality 1 135 sabitha strange nivas 8086336019 No damage 31219021600240 Disbursed to Government 3 Kalpetta Municipality 1 138 manjusha sukrutham nedunilam 7902821756 No damage 31219021600076 Pending THE ADHAR CARD UPDATED ANOTHER ACCOUNT 4 Kalpetta Municipality 1 144 devi krishnan kottachira colony 9526684873 No damage 31219021600129 Verified(LRC Office) NO BRANCH NAME AND IFSC CODE 5 Kalpetta Municipality 1 149 janakiyamma kozhatatta 9495478641 >75% Damage 31219021600080 Verified(LRC Office) PASSBOOK IS NO CLEAR 6 Kalpetta Municipality 1 151 anandavalli kozhathatta 9656336368 No damage 31219021600061 Disbursed to Government 7 Kalpetta Municipality 1 16 chandran nedunilam st colony 9747347814 No damage 31219021600190 Withheld PASSBOOK NOT CLEAR 8 Kalpetta Municipality 1 16 3 sangeetha pradeepan rajasree gives nedunelam 9656256950 No damage 31219021600090 Withheld No damage type details and damage photos 9 Kalpetta Municipality 1 161 shylaja sasneham nedunilam 9349625411 No damage 31219021600074 Disbursed to Government Manjusha padikkandi house 10 Kalpetta Municipality 1 172 3 maniyancode padikkandi house maniyancode 9656467963 16 - 29% Damage 31219021600072 Disbursed to Government 11 Kalpetta Municipality 1 175 vinod madakkunnu colony -

M. R Ry. K. R. Krishna Menon, Avargal, Retired Sub-Judge, Walluvanad Taluk
MARUMAKKATHAYAM MARRIAGE COMMISSION. ANSWERS TO INTERROGATORIES BY M. R RY. K. R. KRISHNA MENON, AVARGAL, RETIRED SUB-JUDGE, WALLUVANAD TALUK. 1. Amongst Nayars and other high caste people, a man of the higher divi sion can have Sambandham with a woman of a lower division. 2, 3, 4 and 5. According to the original institutes of Malabar, Nayars are divided into 18 sects, between whom, except in the last 2, intermarriage was per missible ; and this custom is still found to exist, to a certain extent, both in Travan core and Cochin, This rule has however been varied by custom in British Mala bar, in Avhich a woman of a higher sect is not now permitted to form Sambandham with a man of a lower one. This however will not justify her total excommuni cation from her caste in a religious point of view, but will subject her to some social disabilities, which can be removed by her abandoning the sambandham, and paying a certain fine to the Enangans, or caste-people. The disabilities are the non-invitation of her to feasts and other social gatherings. But she cannot be prevented from entering the pagoda, from bathing in the tank, or touch ing the well &c. A Sambandham originally bad, cannot be validated by a Prayaschitham. In fact, Prayaschitham implies the expiation of sin, which can be incurred only by the violation of a religious rule. Here the rule violated is purely a social one, and not a religious one, and consequently Prayaschitham is altogether out of the place. The restriction is purely the creature of class pride, and this has been carried to such an extent as to pre vent the Sambandham of a woman with a man of her own class, among certain aristocratic families. -
List of Lacs with Local Body Segments (PDF
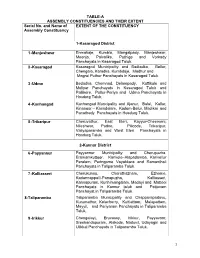
TABLE-A ASSEMBLY CONSTITUENCIES AND THEIR EXTENT Serial No. and Name of EXTENT OF THE CONSTITUENCY Assembly Constituency 1-Kasaragod District 1 -Manjeshwar Enmakaje, Kumbla, Mangalpady, Manjeshwar, Meenja, Paivalike, Puthige and Vorkady Panchayats in Kasaragod Taluk. 2 -Kasaragod Kasaragod Municipality and Badiadka, Bellur, Chengala, Karadka, Kumbdaje, Madhur and Mogral Puthur Panchayats in Kasaragod Taluk. 3 -Udma Bedadka, Chemnad, Delampady, Kuttikole and Muliyar Panchayats in Kasaragod Taluk and Pallikere, Pullur-Periya and Udma Panchayats in Hosdurg Taluk. 4 -Kanhangad Kanhangad Muncipality and Ajanur, Balal, Kallar, Kinanoor – Karindalam, Kodom-Belur, Madikai and Panathady Panchayats in Hosdurg Taluk. 5 -Trikaripur Cheruvathur, East Eleri, Kayyur-Cheemeni, Nileshwar, Padne, Pilicode, Trikaripur, Valiyaparamba and West Eleri Panchayats in Hosdurg Taluk. 2-Kannur District 6 -Payyannur Payyannur Municipality and Cherupuzha, Eramamkuttoor, Kankole–Alapadamba, Karivellur Peralam, Peringome Vayakkara and Ramanthali Panchayats in Taliparamba Taluk. 7 -Kalliasseri Cherukunnu, Cheruthazham, Ezhome, Kadannappalli-Panapuzha, Kalliasseri, Kannapuram, Kunhimangalam, Madayi and Mattool Panchayats in Kannur taluk and Pattuvam Panchayat in Taliparamba Taluk. 8-Taliparamba Taliparamba Municipality and Chapparapadavu, Kurumathur, Kolacherry, Kuttiattoor, Malapattam, Mayyil, and Pariyaram Panchayats in Taliparamba Taluk. 9 -Irikkur Chengalayi, Eruvassy, Irikkur, Payyavoor, Sreekandapuram, Alakode, Naduvil, Udayagiri and Ulikkal Panchayats in Taliparamba -

MODERN INDIAN HISTORY (1857 to the Present)
MODERN INDIAN HISTORY (1857 to the Present) STUDY MATERIAL I / II SEMESTER HIS1(2)C01 Complementary Course of BA English/Economics/Politics/Sociology (CBCSS - 2019 ADMISSION) UNIVERSITY OF CALICUT SCHOOL OF DISTANCE EDUCATION Calicut University P.O, Malappuram, Kerala, India 673 635. 19302 School of Distance Education UNIVERSITY OF CALICUT SCHOOL OF DISTANCE EDUCATION STUDY MATERIAL I / II SEMESTER HIS1(2)C01 : MODERN INDIAN HISTORY (1857 TO THE PRESENT) COMPLEMENTARY COURSE FOR BA ENGLISH/ECONOMICS/POLITICS/SOCIOLOGY Prepared by : Module I & II : Haripriya.M Assistanrt professor of History NSS College, Manjeri. Malappuram. Scrutinised by : Sunil kumar.G Assistanrt professor of History NSS College, Manjeri. Malappuram. Module III&IV : Dr. Ancy .M.A Assistant professor of History School of Distance Education University of Calicut Scrutinised by : Asharaf koyilothan kandiyil Chairman, Board of Studies, History (UG) Govt. College, Mokeri. Modern Indian History (1857 to the present) Page 2 School of Distance Education CONTENTS Module I 4 Module II 35 Module III 45 Module IV 49 Modern Indian History (1857 to the present) Page 3 School of Distance Education MODULE I INDIA AS APOLITICAL ENTITY Battle Of Plassey: Consolodation Of Power By The British. The British conquest of India commenced with the conquest of Bengal which was consummated after fighting two battles against the Nawabs of Bengal, viz the battle of Plassey and the battle of Buxar. At that time, the kingdom of Bengal included the provinces of Bengal, Bihar and Orissa. Wars and intrigues made the British masters over Bengal. The first conflict of English with Nawab of Bengal resulted in the battle of Plassey. -

The Chirakkal Dynasty: Readings Through History
THE CHIRAKKAL DYNASTY: READINGS THROUGH HISTORY Kolathunadu is regarded as one of the old political dynasties in India and was ruled by the Kolathiris. The Mushaka vamsam and the kings were regarded as the ancestors of the Kolathiris. It was mentioned in the Mooshika Vamsa (1980) that the boundary of Mooshaka kingdom was from the North of Mangalapuram – Puthupattanam to the Southern boundary of Korappuzha in Kerala. In the long Sanskrit historical poem Mooshaka Vamsam, the dynastic name of the chieftains of north Malabar (Puzhinad) used is Mooshaka (Aiyappan, 1982). In the beginning of the fifth Century A.D., the kingdom of Ezhimala had risen to political prominence in north Kerala under Nannan… With the death of Nannan ended the most glorious period in the history of the Ezhimala Kingdom… a separate line of rulers known as the Mooshaka kings held sway over this area 36 (Kolathunad) with their capital near Mount Eli. It is not clear whether this line of rulers who are celebrated in the Mooshaka vamsa were subordinate to the Chera rulers of Mahodayapuram or whether they ruled as an independent line of kings on their own right (in Menon, 1972). The narration of the Mooshaka Kingdom up to the 12th Century A.D. is mentioned in the Mooshaka vamsa. This is a kavya (poem) composed by Atula, who was the court poet of the King Srikantha of Mooshaka vamsa. By the 14th Century the old Mooshaka kingdom had come to be known as Kolathunad and a new line of rulers known as the Kolathiris (the ‘Colastri’ of European writers) had come into prominence in north Kerala. -

INFORMATION KERALA MISSION AUDIT REPORT for the PERIOD 1St APRIL 2010 to 31St MARCH 2011 Tr Pinftybflti3££--Ffsng Grt®H¥Ahj & AINELEL CHARTEREDACCOUNTANTS
INFORMATION KERALA MISSION AUDIT REPORT FOR THE PERIOD 1st APRIL 2010 TO 31st MARCH 2011 tr PINftyBflti3££--ffSng grT®H¥AHj & AINELEL CHARTEREDACCOUNTANTS. © SNRA-5, Saphalya Nagar, Kesavadasapuram,Pauh PO , Thiruvanan(hapuram-4 Phone 0471-2557004. Moblle` 9847711005 AUDITORS REPORT We have audited the attached Balance Sheet of Information Kerala Mission, Thiruvananthapuram as at 3]-03-2011 and Income and Expenditure Account and Receipts and Payments Account for the year ended on that date annexed thereto. These financial statements are the responsibility of the management. Our responsibility is to express an opinion on these financial statement based on our audit. We conducted our audit in accordance with auditing standards generally accepted in India. These standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes, examining on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of.financial statements. We believe that our audit provides a reasonable basis for our opinion. We report that : a. We have obtained reasonable information and explanations. which to the best of our knowledge and belief were necessary for the purposes of oiir audit. b. The Balance Sheet, Income and Expenditure AccoLint and Receipts and +lil Payments Account dealt with by this report are in agreement witli the books of account of Information Kerala Mission. c. In our opinion, proper books of account have been kept by Information Kerala Mission so far as appeal.s from our exaiiiination of such books. -

Dictionary of Martyrs: India's Freedom Struggle
DICTIONARY OF MARTYRS INDIA’S FREEDOM STRUGGLE (1857-1947) Vol. 5 Andhra Pradesh, Telangana, Karnataka, Tamil Nadu & Kerala ii Dictionary of Martyrs: India’s Freedom Struggle (1857-1947) Vol. 5 DICTIONARY OF MARTYRSMARTYRS INDIA’S FREEDOM STRUGGLE (1857-1947) Vol. 5 Andhra Pradesh, Telangana, Karnataka, Tamil Nadu & Kerala General Editor Arvind P. Jamkhedkar Chairman, ICHR Executive Editor Rajaneesh Kumar Shukla Member Secretary, ICHR Research Consultant Amit Kumar Gupta Research and Editorial Team Ashfaque Ali Md. Naushad Ali Md. Shakeeb Athar Muhammad Niyas A. Published by MINISTRY OF CULTURE, GOVERNMENT OF IDNIA AND INDIAN COUNCIL OF HISTORICAL RESEARCH iv Dictionary of Martyrs: India’s Freedom Struggle (1857-1947) Vol. 5 MINISTRY OF CULTURE, GOVERNMENT OF INDIA and INDIAN COUNCIL OF HISTORICAL RESEARCH First Edition 2018 Published by MINISTRY OF CULTURE Government of India and INDIAN COUNCIL OF HISTORICAL RESEARCH 35, Ferozeshah Road, New Delhi - 110 001 © ICHR & Ministry of Culture, GoI No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. ISBN 978-81-938176-1-2 Printed in India by MANAK PUBLICATIONS PVT. LTD B-7, Saraswati Complex, Subhash Chowk, Laxmi Nagar, New Delhi 110092 INDIA Phone: 22453894, 22042529 [email protected] State Co-ordinators and their Researchers Andhra Pradesh & Telangana Karnataka (Co-ordinator) (Co-ordinator) V. Ramakrishna B. Surendra Rao S.K. Aruni Research Assistants Research Assistants V. Ramakrishna Reddy A.B. Vaggar I. Sudarshan Rao Ravindranath B.Venkataiah Tamil Nadu Kerala (Co-ordinator) (Co-ordinator) N. -

Tourism-I Indira Gandhi National Open University School of Tourism and Hospitality Services Management
BTMC-131 History of Tourism-I Indira Gandhi National Open University School of Tourism and Hospitality Services Management Block 2 TOURISM: THE CULTURAL HERITAGE UNIT 4 Religions of India 5 UNIT 5 Pilgrimage 21 UNIT 6 Beach and Island Resorts: Kovalam and Lakshadweep 33 COURSE PREPARATION CUM COURSE ADAPTATION TEAM Units 1-3 (TS-1) Units 4-6 (TS-1 & TS-2) Units 18-20 (TS-1 & TS-6) Prof. Kapil Kumar Prof. Kapil Kumar Prof. Kapil Kumar Prof. A.R. Khan Prof. A.R. Khan Prof. A.R. Khan Dr. Ajay Mohurkar Dr. Ajay Mohurkar Dr. Ajay Mohurkar Prof. Swaraj Basu Prof. Swaraj Basu Prof. Swaraj Basu History Faculty History Faculty History Faculty IGNOU IGNOU IGNOU *BTMC – 131 was adopted/adapted from Three Courses, namely – TS-1, TS-2, TS-6 of School of Tourism and Hospitality Services Management (SOTHSM) COURSE PREPARATION AND ADAPTATION TEAM BTMC-131 Prof Jitendra Srivastava, Director, SOTHSM (Chairperson) Dr. Paramita Suklabaidya, Assistant Professor, SOTHSM Dr. Sonia Sharma, Assistant Professor, SOTHSM Dr. Tangjakhombi Akoijam, Assistant Professor, SOTHSM Dr. Arvind Kumar Dubey, Assistant Professor, SOTHSM (Convener) PROGRAMME COORDINATOR COURSE COORDINATOR Dr. Arvind Kumar Dubey Dr. Sonia Sharma Assistant Professor, SOTHSM Assistant Professor, SOTHSM IGNOU, New Delhi Dr. Arvind Kumar Dubey Assistant Professor, SOTHSM BLOCK COORDINATOR: Dr. Sonia Sharma FACULTY MEMBERS Prof. Jitendra Kumar Srivastava Dr. Harkirat Bains Director Associate Professor, SOTHSM Dr. Paramita Suklabaidya Dr. Sonia Sharma Asstt. Prof., SOTHSM Asstt. Prof., SOTHSM Dr. Arvind Kumar Dubey Dr. Tangjakhombi Akoijam Asstt. Prof., SOTHSM Asstt. Prof., SOTHSM PRINT PRODUCTION TYPING ASSISTANCE Mr. Yashpal Mr. Dharmendra Kumar Verma Assistant Registrar (Publication) SOTHSM MPDD, IGNOU, New Delhi January, 2019 Indira Gandhi National Open University, 2019 ISBN : All rights reserved. -

Unit-14 Beach and Island Resorts Kovalam and Lakshadweep.Pdf
UNIT 14 BEACH AND ISLAND RESORTS: KOV ALAM AND LAKSHADWEEP Structure 14.1 Objectives 14.2 Introduction 14.3 Emergence and Growth of Resorts 14.4 Concept of Beach and Island Tourism 14.4.1 Beach Tourism 14.4.2 Island Tourism .14.5 Kovalam Beach Resort 14.5.1 History 14.5.2 Attractions 14.5.3 Ancillary Attractions 1~.5.4 Accommodation and Catering 14.5.5 How to Get There 14.5.6 Other Facilities 14.5.7 Perspective Plan 14.6 Lakshadweep Islands 14.6.1 Geography 14.6.2 History 14.6.3 Attractions 14.6.4 Infrastructure 14.6.5 Accommodation 14.6.6 How to Get There 14.6.7 Tips for Visitors 14.7 Let Us Sum Up 14.8 Key Words . 14.9 Answers to Check Your Progress Exercises 14.1 .OBJECTIVES' After reading this Unit you will be able to: • learn about the emergence and growth of resorts, • understand the concept of beach and island tourism. and • understand the issues involved in the development of beach and island resorts. 14.2 INTRODUCTION Sun, Sea and Sand is the kind of tourism in "n~ue today. You can see that it is coastal zone oriented. The boom in water borne recrec.ion accentuates further the relevance of beach resorts and island resorts in the development of tourism. This Unit first deals with the emergence and growth of resorts. Then it explains the concepts of beach tourism and island tourism. The issues involved in the development of resorts have also been taken up here in the context of the case studies of Lakshad-. -

1 Lakshadweep Islands the Emeralds in Arabian Sea I
_1 LAKSHADWEEP ISLANDS THE EMERALDS IN ARABIAN SEA K.B. Bijurnon Central Institute of Fishcries Nautical and Engineering Training Unit, Chennni Lakshndweep Islands look like emeralds in the vast expanse of blue sea. The codparadise, a tropicsll fantasy awaits with nn unusual, unforgettable and hitfill holiday experience. The r-qral island shaded with palm trees is a memorable experience and the crystal clear water is an abode tbr abundant marine flora and fauna -creeping sea cucumbers, beautiful ornamental fishes, diKcrent species of corals, running hermit crab etc. Historical Backgrozlnd I Early history of Lakshadweep is unwritten. As per the local traditional sayings the first I 1 settlement on these islands were during the period of Cheman Perumal. It is believed that the first 1 settlers were Hindus, as even now the Hindu-social stratification exists in these islands. The advent of Islam dates back to the 17th century around the year 41 Hijra. Tt is believed that one Saint 1 Ubnidullah(r) while praying at Mecca fell asleep. He dreamt that prophet Mohammed wanted him ,I to go to Jeddah, take a ship from there to go distant places. Thus he Icft Jeddah but after sailing for ! I months, a storm wrecked his ship near the small islands, FIoating on a plank he was swept ashore on the island of Amini. From Arnini, St.Wbaidullah arrived at Androth where he met with strong protest but succeeded finally in converting the people to Islam. He next went nearby islands nnd sriccessfully propagated Islm and returned to Andmth where he died and was buried. -

Pazhassi Revolt
MODULE-1 EARLY RESISTANCE AND BRITISH CONSOLIDATION TOPIC- PAZHASSI REVOLT PRIYANKA.E.K ASSISTANT PROFESSOR DEPARTMENT OF HISTORY LITTLE FLOWER COLLEGE, GURUVAYOOR RESISTANCE MOVEMENTS With the establishment of British supremacy, the history of Kerala subjected to changes.(by the Treaty of Srirangapattanam 1792) The period after this can be considered as a period of challenge and response. The British challenge of domination had an equal response from the native chieftains and people. When the British tried to establish their supremacy over the land, resistance against their domination broke out in various parts. The early native resistances were mainly led by the disposed local princes, feudal chieftains, aggrieved peasants, tribal communities and others. The revolts of Pazhassi Raja in Malabar, Paliath Achan in Kochi, the Kurichiyas in Wayanad and the Moplahs in Eranad and Valluvanad are examples of such resistance movements REVOLT OF PATINJARE KOVILAKAM RAJAS Earliest resistance movt was led by some members of the Zamorin’s family Following the withdrawal of Tipu the ruling Zamorin returned from Travancore & formally celebrated his Ariyittuvazhcha in 1792 In the discussions with the Commissioners Zamorins minister demanded the restoration of the former territories to his chief but the company rejected the demand Zamorin entered into a political settlement with the company But this agreement was not approved by the members of Patinjare kovilakam branch of Zamorin family They asserted their independence the senior prince of -

Robert Adams: the Real Founder of English East India Company's
IOSR Journal Of Humanities And Social Science (IOSR-JHSS) Volume 20, Issue 5, Ver. 1 (May. 2015), PP 19-26 e-ISSN: 2279-0837, p-ISSN: 2279-0845. www.iosrjournals.org Robert Adams: the Real Founder of English East India Company’s Supremacy in Malabar Arun Thomas M.,a Dr. Asokan Mundonb a(Research Scholar, Department of History, University of Calicut, Kerala, India- 673635). b(Professor, Department of History, University of Calicut, Kerala, India- 673635). Abstract: The Malabar Coast was famous for its spices particularly pepper, hence got considerable importance throughout the ages. In the age of mercantilism, European Companies searched for the suitable trade centers in Malabar Coast. There were fighting’s among the European Companies for their domination of pepper trade in Malabar. After a long search in late 17th century English Company selected Tellicherry as their trade center. It was well known for the production good quality enormous quantity of pepper that had been in great demand in world markets. In the 18th century, Tellicherry became one of the most considerable and foremost English Company’s settlements of India. The first Chief in Tellicherry factory was Robert Adams. He constructed a powerful platform for English Company in Malabar. There were a number of treatises that deal in the East India Company’s political and economic relations in Malabar, but those works neglected the role of Robert Adams in the establishment of East India Company’s supremacy in Malabar. This study enquires the significance of Robert Adams in the development of English Company’s settlement of Tellicherry. It also aims at how the establishment of Tellicherry factory led to the supremacy of English all over the Malabar region later.