Addis Ababa University School of Graduate Studies Faculty of Life Sciences Zoological Science Programme Unite Ecological and Systematic Zoology Stream
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vulnerability to HIV Infection Among the Borana Pastoral Community of Southern Ethiopia: a Persisting Challenge
Journal name: HIV/AIDS - Research and Palliative Care Article Designation: Original Research Year: 2019 Volume: 11 HIV/AIDS - Research and Palliative Care Dovepress Running head verso: Serbessa Running head recto: Serbessa open access to scientific and medical research DOI: http://dx.doi.org/10.2147/HIV.S193035 Open Access Full Text Article ORIGINAL RESEARCH Vulnerability to HIV infection among the Borana pastoral community of Southern Ethiopia: a persisting challenge Mirgissa Kaba Serbessa Background: Vulnerability to HIV infection is a major concern in an effort to control further infections. What drives vulnerability among pastoral settings of Ethiopia is not well documented. Department of Preventive Medicine, School of Public Health, Addis Ababa Objectives: This study aims to identify drivers of vulnerability to HIV infection among the University, Addis Ababa, Ethiopia Borana pastoral community of Ethiopia. Methods: Data were collected during 2008–2009 as part of a PhD work and subsequently in 2014 and 2016, during a follow-up visit to Borana. Data on perceived threats of HIV, facilita- tors of vulnerability, coping mechanisms and perceived consequences were collected by trained research assistants using topic guides developed for this purpose. In-depth and key informant interviews and Focus Group Discussions (FGDs) with selected married men and women, opinion leaders, and HIV focal persons of public sectors and Non Governmental Organizations in Teltele, Arero, Yabelo and Moyale were carried out. Sample transcripts were checked for consistency and Video abstract completeness before data collection was completed. Two qualitative researchers read transcripts and suggested themes and subthemes in reference to the objective of the study. Transcripts were imported to MAXQDA software. -

Oromia Region Administrative Map(As of 27 March 2013)
ETHIOPIA: Oromia Region Administrative Map (as of 27 March 2013) Amhara Gundo Meskel ! Amuru Dera Kelo ! Agemsa BENISHANGUL ! Jangir Ibantu ! ! Filikilik Hidabu GUMUZ Kiremu ! ! Wara AMHARA Haro ! Obera Jarte Gosha Dire ! ! Abote ! Tsiyon Jars!o ! Ejere Limu Ayana ! Kiremu Alibo ! Jardega Hose Tulu Miki Haro ! ! Kokofe Ababo Mana Mendi ! Gebre ! Gida ! Guracha ! ! Degem AFAR ! Gelila SomHbo oro Abay ! ! Sibu Kiltu Kewo Kere ! Biriti Degem DIRE DAWA Ayana ! ! Fiche Benguwa Chomen Dobi Abuna Ali ! K! ara ! Kuyu Debre Tsige ! Toba Guduru Dedu ! Doro ! ! Achane G/Be!ret Minare Debre ! Mendida Shambu Daleti ! Libanos Weberi Abe Chulute! Jemo ! Abichuna Kombolcha West Limu Hor!o ! Meta Yaya Gota Dongoro Kombolcha Ginde Kachisi Lefo ! Muke Turi Melka Chinaksen ! Gne'a ! N!ejo Fincha!-a Kembolcha R!obi ! Adda Gulele Rafu Jarso ! ! ! Wuchale ! Nopa ! Beret Mekoda Muger ! ! Wellega Nejo ! Goro Kulubi ! ! Funyan Debeka Boji Shikute Berga Jida ! Kombolcha Kober Guto Guduru ! !Duber Water Kersa Haro Jarso ! ! Debra ! ! Bira Gudetu ! Bila Seyo Chobi Kembibit Gutu Che!lenko ! ! Welenkombi Gorfo ! ! Begi Jarso Dirmeji Gida Bila Jimma ! Ketket Mulo ! Kersa Maya Bila Gola ! ! ! Sheno ! Kobo Alem Kondole ! ! Bicho ! Deder Gursum Muklemi Hena Sibu ! Chancho Wenoda ! Mieso Doba Kurfa Maya Beg!i Deboko ! Rare Mida ! Goja Shino Inchini Sululta Aleltu Babile Jimma Mulo ! Meta Guliso Golo Sire Hunde! Deder Chele ! Tobi Lalo ! Mekenejo Bitile ! Kegn Aleltu ! Tulo ! Harawacha ! ! ! ! Rob G! obu Genete ! Ifata Jeldu Lafto Girawa ! Gawo Inango ! Sendafa Mieso Hirna -
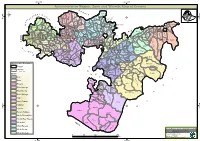
Administrative Region, Zone and Woreda Map of Oromia a M Tigray a Afar M H U Amhara a Uz N M
35°0'0"E 40°0'0"E Administrative Region, Zone and Woreda Map of Oromia A m Tigray A Afar m h u Amhara a uz N m Dera u N u u G " / m r B u l t Dire Dawa " r a e 0 g G n Hareri 0 ' r u u Addis Ababa ' n i H a 0 Gambela m s Somali 0 ° b a K Oromia Ü a I ° o A Hidabu 0 u Wara o r a n SNNPR 0 h a b s o a 1 u r Abote r z 1 d Jarte a Jarso a b s a b i m J i i L i b K Jardega e r L S u G i g n o G A a e m e r b r a u / K e t m uyu D b e n i u l u o Abay B M G i Ginde e a r n L e o e D l o Chomen e M K Beret a a Abe r s Chinaksen B H e t h Yaya Abichuna Gne'a r a c Nejo Dongoro t u Kombolcha a o Gulele R W Gudetu Kondole b Jimma Genete ru J u Adda a a Boji Dirmeji a d o Jida Goro Gutu i Jarso t Gu J o Kembibit b a g B d e Berga l Kersa Bila Seyo e i l t S d D e a i l u u r b Gursum G i e M Haro Maya B b u B o Boji Chekorsa a l d Lalo Asabi g Jimma Rare Mida M Aleltu a D G e e i o u e u Kurfa Chele t r i r Mieso m s Kegn r Gobu Seyo Ifata A f o F a S Ayira Guliso e Tulo b u S e G j a e i S n Gawo Kebe h i a r a Bako F o d G a l e i r y E l i Ambo i Chiro Zuria r Wayu e e e i l d Gaji Tibe d lm a a s Diga e Toke n Jimma Horo Zuria s e Dale Wabera n a w Tuka B Haru h e N Gimbichu t Kutaye e Yubdo W B Chwaka C a Goba Koricha a Leka a Gidami Boneya Boshe D M A Dale Sadi l Gemechis J I e Sayo Nole Dulecha lu k Nole Kaba i Tikur Alem o l D Lalo Kile Wama Hagalo o b r Yama Logi Welel Akaki a a a Enchini i Dawo ' b Meko n Gena e U Anchar a Midega Tola h a G Dabo a t t M Babile o Jimma Nunu c W e H l d m i K S i s a Kersana o f Hana Arjo D n Becho A o t -

Ethiopia: 3W - Health Cluster Ongoing Activities Map (December 2016)
Ethiopia: 3W - Health Cluster Ongoing Activities map (December 2016) ERITREA 8 Total Number of Partners Ahferom CCM CCM GOAL GOAL Erob CCM Adwa GOAL Red Sea GOAL Werei CCM Leke GOAL Koneba GOAL Hawzen GOAL CCM SUDAN TIGRAY GOAL Ab Ala GOAL AMHARA Megale Gulf of GOAL Aden DCA IMC Kobo AFAR Lay DCA Meket DCA Gayint IMC IMC Tach Gayint DCA Guba Lafto GOAL BENESHANGUL Dera IMC Worebabu Simada GOAL GOAL GOAL GOAL GUMU IMC Thehulederie Sirba DJIBOUTI Abay Telalak Afambo GOAL GOAL IRC Tenta GOAL Sayint GOAL GOAL IRC GOAL GOAL Were Ilu Ayisha IRC IRC GOAL Dewa Sherkole Legehida Harewa Kurmuk GOAL IMC Menge Kelela Artuma IRC Yaso Fursi IMC Erer IRC IRC IRC Jille Menz IMC Timuga Dembel Wara Afdem Bilidigilu IRC Mama Assosa IRC Jarso IMC Tarema IMC Midir Ber IRC IRC Agalometi Gerar IMC Jarso Kamashi IMC Bambasi GOAL DIRE Chinaksen IMC IMC IRC IMC DAWA IMC Bio Jiganifado Ankober Meta IRC GOAL IRC IMC IMC Aleltu Deder HARERI GOAL GOAL Gursum IRC IRC IRC GOAL Midega SOMALIA IRC IMC Goba SOUTH SUDAN Tola ACF Koricha Anfilo IMC Gashamo Anchar GOAL Daro Lebu Boke Golo Oda IRC Wantawo GOAL Meyu IMC IRC IRC IRC GOAL GOAL IMC Aware SCI IMC Fik IRC IRC Kokir Sire Jikawo IRC Gedbano Adami IMC GOAL Tulu Jido Degehabur GOAL SCI GOAL Sude Akobo Selti Kombolcha IRC IRC Lanfero Hamero Gunagado Mena Dalocha IMC GAMBELA GOAL Arsi IMC Shekosh GOAL Gololcha GOAL Negele Bale IMC Soro GOAL IMC IRC GOAL IMC Agarfa IRC Tembaro IRC IRC GOAL SCI GOAL GOAL IMC IMC Ginir CCM GOAL GOAL IRC IMC IMC GOAL GOAL IRC GOAL Sinana IMC IRC IRC Dinsho GOAL Goba IRC IMC GOAL IRC GOAL IRC Adaba CCM GOAL Berbere IMC Humbo GOAL SOMALI IMC Hulla IRC GOAL CCM GOAL GOAL GOAL PIN IRC Zala IMC IRC IRC Abaya PIN IRC Wenago Ubadebretsehay Mirab Gelana Abaya IRC GOAL GOAL SCI IRC IRC SCI Amaro OROMIA SNNPR IRC SCI CCM Bonke GOAL IRC Meda CCM SCI Welabu Legend SCI Konso IMC SCI International boundary Filtu Hudet INDIAN Agencies' locOaCtiEoAnNs and Regional boundary SCI Arero Dolobay Dolo Odo area of interventions are IMC No. -

Report on General Characteristics of the Borana Zone, Ethiopia
IVM Institute for Environmental Studies 7 Report on general characteristics of the Borana zone, Ethiopia R. Lasage, A. Seifu, M. Hoogland, A. de Vries Report R-10/03 18 November 2010 This report was commissioned by: The ADAPTS project It was internally reviewed by: Pieter Pauw IVM Action for Development (AfD) Institute for Environmental Studies P.O. Box 19859 VU University Amsterdam Addis Abeba, Ethiopië De Boelelaan 1087 Acacia Water 1081 HV AMSTERDAM Jan van Beaumontstraat 1 T +31-20-598 9555 2805 RN Gouda F +31-20-598 9553 E [email protected] Both Ends Nieuwe Keizersgracht 45 1018 VC Amsterdam Copyright © 2010, Institute for Environmental Studies All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photo- copying, recording or otherwise without the prior written permission of the copyright holder IVM Institute for Environmental Studies Report on general characteristics of the Borana zone, Ethiopia Contents 1 Introduction 5 2 General characteristics 7 3 Physical characteristics 9 3.1 Current climate 9 3.2 Geology and soils 11 3.3 Hydrology 12 Traditional water sources 12 b Modern/Improved water sources 14 4 Socio-economical characteristics 19 4.1 Population 19 4.2 Traditional Social Set up and the Geda System in Borana 19 4.3 Community and Livestock Mobility 20 4.4 Economic situation 22 Community Economic Survey – Proportional Piling 22 5 Institutional characteristics 27 5.1 Zonal level 28 a. Modern Water Management System 29 b. Traditional Water Management System 30 References 33 Annex A 35 IVM Institute for Environmental Studies Report on general characteristics of the Borana zone, Ethiopia 5 1 Introduction This report combines the general information that was gathered in the context of the ADAPTS project for the case study in Borana, Ethiopia. -

Pdf | 148.78 Kb
United Nations Nations Unies Office for the Coordination of Bureau de Coordination des Humanitarian Affairs in Ethiopia Affaires Humanitaires au Ethiopie Website: Website: http://ochaonline.un.org/ethiopia http://ochaonline.un.org/ethiopia SITUATION REPORT: EXTENDED DRY SEASON IN LOWLAND AGRO-PASTORAL AREAS OF OROMIYA REGION1 – 11 MARCH 2008 The deteriorating situation with regard to human health, food security, livelihoods, and livestock health initially reported in Borena zone has spread to Bale, East Hararghe, Guji and Liben zones of Oromiya Region. Poorly performing rains for the upcoming rainy season forecasted by the National Meteorological Agency are likely to exacerbate the existing situation in the lowland agro-pastoral areas of Oromiya Region. In addition to the situation in Oromiya Region, UN agencies and humanitarian partners have reported the emergence of hotspots in SNNPR in the following zones; Gamogofa, Hadiya, Kenbata, Sidama, South Omo and Welayita. An estimated 88,000 people in affected woredas in Borena zone require emergency assistance from government, humanitarian partners and UN agencies. This estimate is expected to be revised when the requirements of communities in Arero, Teltele and Yabello are taken into consideration. Recent field assessments have identified additional beneficiaries in Bale and Guji 1 This situation report is based on information gathered from a variety of sources including; UN agencies, NGO and government partners. zones of Oromiya Region who will require emergency food assistance over the next four months. WFP field officers on the ground report rapid deterioration of the food security situation in East Hararghe zone with serious food shortages in Midhega Tola, Chenaksen, Kurfa Chelle, Metta and Bedeno woredas. -

ETHIOPIA Food Security Outlook Update November 2010 Poor Water
ETHIOPIA Food Security Outlook Update November 2010 Poor water availability in the southeast following below-average Oct-Dec rains Key Messages Figure 1. Estimated food security outcomes, October to December 2010 To date, the performance of hageya/deyr rains has been below average as predicted. This has resulted in shortages of pasture and water in the southeastern pastoral and agropastoral parts of the country. Land preparation and planting of transitional crops, mainly sweet potato has been carried out as usual in SNNPR. Performance of these crops will highly depend on the performance of the sapie rains in December that are predicted to be normal to below normal this year. Overall meher season crop harvests (October to January) are expected to be normal to above normal this year, except in areas that were affected by water logging, floods, landslides, dry spells and yellow wheat rust, resulting in an Source: FEWS NET and WFP overall improvement in food security in dominantly meher Figure 2. Estimated food security outcomes, January crop producing parts of the country. to March 2011 Updated food security outlook through March 2011 The period November to March is typically a time of stable food security in Ethiopia given the meher harvest (October to January) which contributes 90 to 95 percent of total annual crop production. With the start of the harvest, staple food prices typically decline, further contributing to the improvement of food security. This year, the overall performance of meher season crops has been average to above average, although localized shocks led to reductions in production in some areas. -

ETHIOPIA Food Security Alert February 11, 2008
ETHIOPIA Food Security Alert February 11, 2008 Poor rain forecasts suggest increased food insecurity The Government of Ethiopia’s (GoE) National Meteorology Figure 1. Climate outlook for the 2008 gu/ganna/belg Agency forecasts below‐normal 2008 gu/ganna/belg rains season (March to May) (March to May) in the eastern half of the country. These rains are critical in southeastern pastoral areas of Somali Region and neighboring lowlands of Borena, Guji, and Bale zones of Oromiya Region, where households are already highly food insecure due to the poor performance of the 2007 gu and deyr (October to December) rains. Poor or failed gu rains in 2008 will likely lead to extreme food insecurity in these areas, unless immediate interventions are implemented. Contingency planning for such interventions is required immediately. In addition to poor 2007 rains, restrictions on trade and movement in Somali Region that began in June 2007 have severely limited market access for pastoral and agropastoral populations living in Gode, Korahe, Degehabur, Warder, and Source: Based on National Meteorological Agency’s January 2008 Climate Kebridehar zones. While slight improvements have been Outlook forum; Graphics by FEWS NET noted in the movement of commercial cereals to these zones, livestock exports – the main source of income for most of populations in these areas – continue to be limited. This has made it difficult for people to purchase food, which has become expensive due, in part, to the restrictions. Abnormal livestock migrations, critical levels of acute malnutrition and measles occurrences have been reported in some of these areas. Poor October to November rains in parts of Borena, Guji, and Bale zones of Oromiya Region led to earlier‐than‐normal depletion of pasture and water resources, particularly in Dire, Moyale, Miyo Dillo, Dhas, Arero, Yabello, and Teltele woredas (districts) of Borena Zone. -

Download File
TECHNICAL REPORT PATHWAYS TO PEACE SERIES: ADDRESSING CONFLICT AND STRENGTHENING STABILITY IN A CHANGING CLIMATE LESSONS LEARNED FROM THE PEACE CENTERS FOR CLIMATE AND SOCIAL RESILIENCE PROJECT Updated June 2019 This document was produced for review by the United States Agency for International Development. It was prepared by Jeffrey Stark, Katsuaki Terasawa and Chalachew Niguse Agonafir for the Adaptation Thought Leadership and Assessments (ATLAS) Task Order No. AID-OAA-I-14-00013, under the Restoring the Environment through Prosperity, Livelihoods, and Conserving Ecosystems (REPLACE) IDIQ. Chemonics contact: Chris Perine, Chief of Party ([email protected])- Chemonics International Inc. 1717 H Street NW Washington, DC 20006 ATLAS reports and other products are available on the Climatelinks website: https://www.climatelinks.org/projects/atlas Cover Photo: Katsuaki Terasawa, Yabelo District, Oromia National Regional State, Ethiopia, 2017. Herders move livestock in search of water and pasture. In Oromia, more frequent and severe droughts, amplified by climate change, are reducing water supplies, forcing herders to travel longer distances and contributing to conflict among pastoralists. Peacebuilding initiatives that address both climate and non-climate stressors are helping reduce violence and promote peace. PATHWAYS TO PEACE SERIES: ADDRESSING CONFLICT AND STRENGTHENING STABILITY IN A CHANGING CLIMATE LESSONS LEARNED FROM THE PEACE CENTERS FOR CLIMATE AND SOCIAL RESILIENCE PROJECT Updated June 2019 Prepared for: United States Agency for International Development Adaptation Thought Leadership and Assessments (ATLAS) Prepared by: Chemonics International Inc. Jeffrey Stark Katsuaki Terasawa Chalachew Niguse Agonafir This report is made possible by the support of the American People through the United States Agency for International Development (USAID). -

Bi-Monthly Prioritization of Shelter/NFI Needs End of August 2017
Bi-monthly prioritization of shelter/NFI needs End of August 2017 Life-saving nature of shelter/NFI assistance Shelter is a basic human need and a critical determinant for survival and coping in the majority of crises. Beyond survival, shelter and core relief items are necessary to provide security and ensure personal safety and protection, and to promote resistance to ill-health and disease. Response activities As of 30 August 2017: 57,564 full emergency shelter & NFI kits distributed to displaced households in 6 regions, additionally to cash grants and vouchers to 982 households. 2,878 supplementary kits presently being distributed or already funded and procured for distributions. 18,400 kits in stock & pipe-line. TOTAL = 79,824 full kits or equivalent in cash and vouchers (61%) against the 131,500 kits MYR target (Gap: 51,676 kits – 39%). Remaining needs The DTM round 6 figures collected in 7 regions, the 2017 Belg assessments conducted in 4 regions, requests received from the authorities including the needs induced by the 2017 kiremt season as shared by the flood task force and assessments conducted by cluster members revealed that the total of households in need of shelter/NFI assistance is 172,097 HHs. Prioritization criteria As agreed upon among cluster members on 31 of August 2017, Shelter/NFI assistance for people who are currently in need of assistance will primarily be directed to: Newly displaced in 2017, IDPs who have not had access to shelter/NFI assistance, durable solutions or coping mechanisms and Populations affected -

Extended Dry Season in Borena Zone1 – 29 February 2008
United Nations Nations Unies Office for the Coordination of Bureau de Coordination des Affaires Humanitarian Affairs in Ethiopia Humanitaires au Ethiopie Website: Website: http://ochaonline.un.org/ethiopia http://ochaonline.un.org/ethiopia SITUATION REPORT: EXTENDED DRY SEASON IN BORENA ZONE1 – 29 FEBRUARY 2008 The situation in Borena zone with regard to animal health, food security and water for human and animal consumption is deteriorating on a daily basis. The extended dry season follows insufficient rainfall during the hagaya rain (October-December) and conditions have been exacerbated by overstocking of livestock and encroachment of land by farms and bush trees. Coping mechanisms are stretched to breaking point and pastoralist communities, children, the elderly and people living with HIV/AIDS are particularly vulnerable to livelihood and health risks. While conditions in Borena do not currently fall under classification of a full scale drought there is a risk that poor performance of the upcoming ganna rains will have a serious impact upon human health, animal welfare and livelihoods in the region. Prompt and coordinated intervention by government and humanitarian partners could limit the impact upon human life, health and livelihoods in the region. Priority needs are water for human and livestock consumption, animal feed provision to mother cows and calves and animal health services. Specific programmatic 1 This situation report is based on information gathered from a variety of sources including; UN agencies, NGO and government -

ETHIOPIA Humanitarian Access Situation Report July - September 2020
ETHIOPIA Humanitarian Access Situation Report July - September 2020 This report is produced by OCHA Ethiopia in collaboration with humanitarian partners. It covers the period July to September 2020. The next report will be issued in December 2020. Highlights ! ! ! ! ! ! ! ! ! ! ! • Ethiopia’s humanitarian and access situation has ! ! ! ! ! ! ! ! ! ! ! !! ! ! ! ! ! ! North! Western ! Eastern ! ! ! ! ! ! Central ! ! ! ! ! ! !! ! ! ! worsened due to COVID-19, increased food insecurity ! ! ! ! Western ! ! ! ! ! ! ! ! ! ! ! TIGRAY ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Mekele! Special ! ! ! Zone 2 (Kilbet Rasu) ! ! ! ! ! ! ! ! ! ! ! ! ! ! South Eastern ! North Gondar ! due to desert locust, and new forced displacements ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! related to armed conflict, inter-communal violence, Wag Hamra ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Southern ! ! Central Gondar ! ! ! ! ! ! ! ! ! ! ! AFAR ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Zone 4 (Fantana Rasu) ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Number of incidents by woreda ! ! ! ! and social unrest, particularly in the Oromia region. ! ! West Gondar ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! North Wello ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !