Diversity of Plants Monophyly
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Photosynthetic Eukaryotes Inhabited Low-Salinity Habitats
Early photosynthetic eukaryotes inhabited PNAS PLUS low-salinity habitats Patricia Sánchez-Baracaldoa,1, John A. Ravenb,c, Davide Pisanid,e, and Andrew H. Knollf aSchool of Geographical Sciences, University of Bristol, Bristol BS8 1SS, United Kingdom; bDivision of Plant Science, University of Dundee at the James Hutton Institute, Dundee DD2 5DA, United Kingdom; cPlant Functional Biology and Climate Change Cluster, University of Technology Sydney, Ultimo, NSW 2007, Australia; dSchool of Biological Sciences, University of Bristol, Bristol BS8 1TH, United Kingdom; eSchool of Earth Sciences, University of Bristol, Bristol BS8 1TH, United Kingdom; and fDepartment of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138 Edited by Peter R. Crane, Oak Spring Garden Foundation, Upperville, Virginia, and approved July 7, 2017 (received for review December 7, 2016) The early evolutionary history of the chloroplast lineage remains estimates for the origin of plastids ranging over 800 My (7). At the an open question. It is widely accepted that the endosymbiosis that same time, the ecological setting in which this endosymbiotic event established the chloroplast lineage in eukaryotes can be traced occurred has not been fully explored (8), partly because of phy- back to a single event, in which a cyanobacterium was incorpo- logenetic uncertainties and preservational biases of the fossil re- rated into a protistan host. It is still unclear, however, which cord. Phylogenomics and trait evolution analysis have pointed to a Cyanobacteria are most closely related to the chloroplast, when the freshwater origin for Cyanobacteria (9–11), providing an approach plastid lineage first evolved, and in what habitats this endosym- to address the early diversification of terrestrial biota for which the biotic event occurred. -

Gymnosperms the MESOZOIC: ERA of GYMNOSPERM DOMINANCE
Chapter 24 Gymnosperms THE MESOZOIC: ERA OF GYMNOSPERM DOMINANCE THE VASCULAR SYSTEM OF GYMNOSPERMS CYCADS GINKGO CONIFERS Pinaceae Include the Pines, Firs, and Spruces Cupressaceae Include the Junipers, Cypresses, and Redwoods Taxaceae Include the Yews, but Plum Yews Belong to Cephalotaxaceae Podocarpaceae and Araucariaceae Are Largely Southern Hemisphere Conifers THE LIFE CYCLE OF PINUS, A REPRESENTATIVE GYMNOSPERM Pollen and Ovules Are Produced in Different Kinds of Structures Pollination Replaces the Need for Free Water Fertilization Leads to Seed Formation GNETOPHYTES GYMNOSPERMS: SEEDS, POLLEN, AND WOOD THE ECOLOGICAL AND ECONOMIC IMPORTANCE OF GYMNOSPERMS The Origin of Seeds, Pollen, and Wood Seeds and Pollen Are Key Reproductive SUMMARY Innovations for Life on Land Seed Plants Have Distinctive Vegetative PLANTS, PEOPLE, AND THE Features ENVIRONMENT: The California Coast Relationships among Gymnosperms Redwood Forest 1 KEY CONCEPTS 1. The evolution of seeds, pollen, and wood freed plants from the need for water during reproduction, allowed for more effective dispersal of sperm, increased parental investment in the next generation and allowed for greater size and strength. 2. Seed plants originated in the Devonian period from a group called the progymnosperms, which possessed wood and heterospory, but reproduced by releasing spores. Currently, five lineages of seed plants survive--the flowering plants plus four groups of gymnosperms: cycads, Ginkgo, conifers, and gnetophytes. Conifers are the best known and most economically important group, including pines, firs, spruces, hemlocks, redwoods, cedars, cypress, yews, and several Southern Hemisphere genera. 3. The pine life cycle is heterosporous. Pollen strobili are small and seasonal. Each sporophyll has two microsporangia, in which microspores are formed and divide into immature male gametophytes while still retained in the microsporangia. -

Algae & Marine Plants of Point Reyes
Algae & Marine Plants of Point Reyes Green Algae or Chlorophyta Genus/Species Common Name Acrosiphonia coalita Green rope, Tangled weed Blidingia minima Blidingia minima var. vexata Dwarf sea hair Bryopsis corticulans Cladophora columbiana Green tuft alga Codium fragile subsp. californicum Sea staghorn Codium setchellii Smooth spongy cushion, Green spongy cushion Trentepohlia aurea Ulva californica Ulva fenestrata Sea lettuce Ulva intestinalis Sea hair, Sea lettuce, Gutweed, Grass kelp Ulva linza Ulva taeniata Urospora sp. Brown Algae or Ochrophyta Genus/Species Common Name Alaria marginata Ribbon kelp, Winged kelp Analipus japonicus Fir branch seaweed, Sea fir Coilodesme californica Dactylosiphon bullosus Desmarestia herbacea Desmarestia latifrons Egregia menziesii Feather boa Fucus distichus Bladderwrack, Rockweed Haplogloia andersonii Anderson's gooey brown Laminaria setchellii Southern stiff-stiped kelp Laminaria sinclairii Leathesia marina Sea cauliflower Melanosiphon intestinalis Twisted sea tubes Nereocystis luetkeana Bull kelp, Bullwhip kelp, Bladder wrack, Edible kelp, Ribbon kelp Pelvetiopsis limitata Petalonia fascia False kelp Petrospongium rugosum Phaeostrophion irregulare Sand-scoured false kelp Pterygophora californica Woody-stemmed kelp, Stalked kelp, Walking kelp Ralfsia sp. Silvetia compressa Rockweed Stephanocystis osmundacea Page 1 of 4 Red Algae or Rhodophyta Genus/Species Common Name Ahnfeltia fastigiata Bushy Ahnfelt's seaweed Ahnfeltiopsis linearis Anisocladella pacifica Bangia sp. Bossiella dichotoma Bossiella -

The Moss-Back Alga (Cladophorophyceae, Chlorophyta) on Two Species of Freshwater Turtles in the Kimberleys
Telopea 12(2) 279–284 The moss-back alga (Cladophorophyceae, Chlorophyta) on two species of freshwater turtles in the Kimberleys Stephen Skinner1,2, Nancy FitzSimmons3 and Timothy J. Entwisle1 1National Herbarium of New South Wales, Mrs Macquaries Road, Sydney NSW 2000 Australia 2Southern ACT Catchment Group Inc., PO Box 2056, Kambah, ACT Author for correspondence: [email protected] 3Institute for Applied Ecology, School of Resource, Environmental & Heritage Sciences, University of Canberra, Canberra, ACT 2601, Australia Abstract The range of the Australian freshwater alga Basicladia ramulosa Ducker is extended, both in its turtle hosts (Chelodina burrungandjii Thomson et al.; Emydura australis (Grey)) and in geography, to tropical northern Western Australia. Along with further morphological observations, sporangia are described for the first time in this taxon. Introduction Moss-back turtles (Fig. 1) have fascinated biologists for many years. While the carapace of a potentially amphibious turtle would be a challenging habitat for most aquatic organisms, it is perhaps surprising there are only a handful of attached algae reported from such sites. Edgren et al. (1953) detailed the range of host turtles then known in North America and the range of epizoic algae that included Rhizoclonium and Cladophora. Two further genera in the Cladophoraceae are the only macroalgae widely reported on turtle carapaces: the prostrate, spreading, endozoic (and possibly disease causing) Dermatophyton radicans Peter, and species of the heterotrichous genus Basicladia, responsible for the name ‘moss-back’. In the United States, Basicladia is considered a small epizoic genus on turtles and water snails, of three to four taxa (John 2003). Hamilton (1948) described sexual reproduction in North American species of Basicladia involving the fusion of biflagellate zooids as is commonly the case in the Cladophoraceae. -
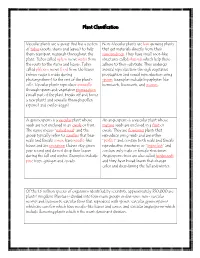
Plant Classification
Plant Classification Vascular plants are a group that has a system Non-Vascular plants are low growing plants of tubes (roots, stems and leaves) to help that get materials directly from their them transport materials throughout the surroundings. They have small root-like plant. Tubes called xylem move water from structures called rhizoids which help them the roots to the stems and leaves. Tubes adhere to their substrate. They undergo called phloem move food from the leaves asexual reproduction through vegetative (where sugar is made during propagation and sexual reproduction using photosynthesis) to the rest of the plant’s spores. Examples include bryophytes like cells. Vascular plants reproduce asexually hornworts, liverworts, and mosses. through spores and vegetative propagation (small part of the plant breaks off and forms a new plant) and sexually through pollen (sperm) and ovules (eggs). A gymnosperm is a vascular plant whose An angiosperm is a vascular plant whose seeds are not enclosed in an ovule or fruit. mature seeds are enclosed in a fruit or The name means “naked seed” and the ovule. They are flowering plants that group typically refers to conifers that bear reproduce using seeds and are either male and female cones, have needle-like “perfect” and contain both male and female leaves and are evergreen (leaves stay green reproductive structures or “imperfect” and year round and do not drop their leaves contain only male or female structures. during the fall and winter. Examples include Angiosperm trees are also called hardwoods pine trees, ginkgos and cycads. and they have broad leaves that change color and drop during the fall and winter. -

B.Sc Botany (Sub.) I Group: a General Characters of Gymnosperm
1 B.Sc Botany (Sub.) I Group: A General Characters of Gymnosperm Gymnosperms are a small group of plants comprising only 70 genera and 725 living species. The word Gymnosperm was used by the Greek botanists Theophrastus in 300 B.C. for the plants with unprotected seeds. Gymnosperms are naked seeded plants. The gymnosperms are characterized by the following features: i. It shows the distinct alternation of generations. ii. Sporophytic generation is the dormant phase of the life cycle. The main plants are sporophyte and the gametophytes are dependent on it throughout. iii. The sporophytic plants are usually tall, woody, perennial trees or shrubs and differentiated into root, stem and leaves. iv. Root- Root is usually well developed tap root system. The stele in root is diarch or polyarch. v. Stem- stems are usually branched but unbranched in Cycas. vi. The leaves may be compound as in Cycas or simple as in Pinus. Internal structure: i. The vascular bundles in stem are arranged in a ring. The bundles are conjoint, collateral and open. ii. The secondary growth is effected by a cambial layer which produces secondary xylem and secondary phloem on the inner and outer side respectively. iii. The secondary wood forming distinct annual ring. Spring wood: It is made up of layer tracheids with the walls and layer lumen. Autumn wood: It is made up of compactly arranged smaller tracheids with thick walls and smaller lumen. iv. The wood may be manoxylic or pycnoxylic and may be monoxylic or polyxylic. Dr. Sanjeev Kumar Vidyarthi, dept. of Botany, Dr. L.K.V.D. -

Dr. Mitch Pavao-Zuckerman Department of Ecology and Evolutionary Biology
Dr. Mitch Pavao-Zuckerman Department of Ecology and Evolutionary Biology 621621--82208220 mzuckermzucker@[email protected] OfficeOffice hours:hours: BiosciencesBiosciences WestWest 431431 WW andand FF 11--22 p.m.p.m. oror byby appointmentappointment Diversity of Plants Diversity of Plants (Fig 29.4) Chlorophyta Ancestral Alga Nontracheophytes Nonseed Tracheophytes Gymnosperms The Transition to Life on Land Angiosperms The Vascular Plants The Seed Plants The Flowering Plants Monophyly • Monophyletic group – includes the most recent common ancestor and all decendents • These are NOT monophyletic: GreenGreen PlantsPlants ((viridiphytesviridiphytes)) areare aa monophyleticmonophyletic groupgroup • Green Plants include the Chlorophytes (green algae) • Other green algae • and the land plants EmbryophytesEmbryophytes (Land(Land Plants)Plants) Land Plants are also a monophyletic group • Photosynthetic eukaryotes that use chlorophyll a and b and store carbohydrates starch • Resting embryo with placental connection to the parent. The Conquest of the Land HistoryHistory ofof plantsplants onon landland •• 500500 myamya -- aa fewfew algaealgae andand lichens.lichens. •• ByBy 460460 myamya -- primitiveprimitive LandLand PlantsPlants,, •• ByBy 425425 myamya -- EarlyEarly VascularVascular PlantsPlants werewere commoncommon •• HowHow diddid itit happen?happen? •• Obstacles?Obstacles? Reconstruction Fossil The Conquest of the Land EarlyEarly innovationsinnovations inin landland plantplant evolution:evolution: 1.1. cuticlecuticle (waxy(waxy -

The Marine Species of Cladophora (Chlorophyta) from the South African East Coast
NovaHedwigia 76 1—2 45—82 Stuttgart, Februar 2003 The marine species of Cladophora (Chlorophyta) from the South African East Coast by F. Leliaert and E. Coppejans Research Group Phycology, Department of Biology, Ghent University, Krijgslaan 281, S8 B-9000 Ghent, Belgium E-mails: [email protected] and [email protected] With 16 figures and 5 tables Leliaert, F. & E. Coppejans (2003): The marine species of Cladophora (Chlorophyta) from the South African East Coast. - Nova Hedwigia 76: 45-82. Abstract: Twelve species of the genus Cladophora occur along the South African East Coast. Detailed descriptions and illustrations are presented. Four species are recorded for the first time in South Africa: C. catenata , C. vagabunda , C. horii and C. dotyana; the last two are also new records for the Indian Ocean. A comparison of the South African C. rugulosa specimens with specimens of C. prolifera from South Africa and other regions have shown that these species are not synonymous as previously considered, leading to the resurrection of C. rugulosa which is probably a South African endemic. Key words: Cladophora, C. catenata , C. dotyana, C. horii, C. prolifera , C. rugulosa , C. vagabunda , South Africa, KwaZulu-Natal. Introduction Cladophora Kützing is one of the largest green-algal genera and has a worldwide distribution. Within the class Cladophorophyceae the genus Cladophora is characterized by its simple thallus architecture: branched, uniseriate filaments of multinucleate cells. Eleven different architectural types (sections) are distinguished in the genus (van den Hoek 1963, 1982; van den Hoek & Chihara 2000). Recent studies based on morphological and molecular data have proven that Cladophora is polyphyletic (van den Hoek 1982; Bakker et al. -

Monitoring of Biofouling Communities in a Portuguese Port Using a Combined Morphological and Metabarcoding Approach Joana Azevedo 1,2,3, Jorge T
www.nature.com/scientificreports OPEN Monitoring of biofouling communities in a Portuguese port using a combined morphological and metabarcoding approach Joana Azevedo 1,2,3, Jorge T. Antunes1,2,3, André M. Machado 1, Vitor Vasconcelos1,2, Pedro N. Leão 1* & Elsa Froufe 1* Marine biofouling remains an unsolved problem with a serious economic impact on several marine associated industries and constitutes a major vector for the spread of non-indigenous species (NIS). The implementation of biofouling monitoring programs allows for better fouling management and also for the early identifcation of NIS. However, few monitoring studies have used recent methods, such as metabarcoding, that can signifcantly enhance the detection of those species. Here, we employed monthly monitoring of biofouling growth on stainless steel plates in the Atlantic Port of Leixões (Northern Portugal), over one year to test the efect of commercial anti-corrosion paint in the communities. Fouling organisms were identifed by combining morpho-taxonomy identifcation with community DNA metabarcoding using multiple markers (16S rRNA, 18S rRNA, 23S rRNA, and COI genes). The dominant colonizers found at this location were hard foulers, namely barnacles and mussels, while other groups of organisms such as cnidarians, bryozoans, and ascidians were also abundant. Regarding the temporal dynamics of the fouling communities, there was a progressive increase in the colonization of cyanobacteria, green algae, and red algae during the sampled period with the replacement of less abundant groups. The tested anticorrosion paint demonstrated to have a signifcant prevention efect against the biofouling community resulting in a biomass reduction. Our study also reports, for the frst time, 29 NIS in this port, substantiating the need for the implementation of recurring biofouling monitoring programs in ports and harbours. -

A Global Analysis of the Distribution and Conservation Status Of
Journal of Biogeography (J. Biogeogr.) (2015) 42, 809–820 SYNTHESIS Fighting their last stand? A global analysis of the distribution and conservation status of gymnosperms Yann Fragniere1,Sebastien Betrisey2,3,Leonard Cardinaux1, Markus Stoffel4,5 and Gregor Kozlowski1,2* 1Natural History Museum Fribourg, CH-1700 ABSTRACT Fribourg, Switzerland, 2Department of Biology Aim Gymnosperms are often described as a marginal and threatened group, and Botanic Garden, University of Fribourg, members of which tend to be out-competed by angiosperms and which therefore CH-1700 Fribourg, Switzerland, 3Conservation Biogeography, Department of preferentially persist at higher latitudes and elevations. The aim of our synthesis Geosciences, University of Fribourg, CH-1700 was to test these statements by investigating the global latitudinal and elevational Fribourg, Switzerland, 4Dendrolab.ch, distribution of gymnosperms, as well as their conservation status, using all extant Institute of Geological Sciences, University of gymnosperm groups (cycads, gnetophytes, ginkgophytes and conifers). 5 Bern, CH-3012 Bern, Switzerland, Institute Location Worldwide. for Environmental Sciences, Climatic Change and Climate Impacts, University of Geneva, Methods We developed a database of 1014 species of gymnosperms containing CH-1227 Carouge, Switzerland latitudinal and elevational distribution data, as well as their global conservation status, as described in the literature. The 1014 species comprised 305 cycads, 101 gnetophytes, the only living representative of ginkgophytes, and 607 conifers. Generalized additive models, frequency histograms, kernel density estimations and distribution maps based on Takhtajan’s floristic regions were used. Results Although the diversity of gymnosperms decreases at equatorial lati- tudes, approximately 50% of the extant species occur primarily between the tropics. More than 43% of gymnosperms can occur at very low elevations (≤ 200 m a.s.l.). -

Name That Gymnosperm
A B C D This tree is found frequently This evergreen is found throughout This species is found at drier and This Wyoming tree is a remnant of throughout the eastern half of the state. The species is known for lower elevations compared to some the last ice age and is found exclu- Wyoming ranging from the Black often having serotinous cones that of the other trees pictured. This tree sively in the Black Hills of Wyoming Hills to the Laramie Mountains and only open to release seeds when the shares its name with the town in and South Dakota. It grows at higher the Bighorns. The tree is known cone is heated. Once the limbs are central Wyoming. elevations along riparian or wet areas for its ability to withstand the heat removed, this tree makes an excel- in its native habitat. of wildfires because of its thick, lent pole for teepees because of its reddish-colored bark. long, slender trunk. NAME THAT GYMNOSPERM Wyoming is host to many conifer (gymnosperm or “naked in hot, dry, and low elevations while others are found in cold and seed” plants including conifer, cycads, and ginkos) tree species. high elevations. Cones are an excellent tool that can be used for The cones in this quiz and their parent trees are found at different identification of evergreens. Match the tree to the photo. Good geographical locations across the Cowboy State. Some are found luck and keep an eye out for these trees this year! E F G H Known for having extremely flexible This species is probably better known This tree is found in most high- This tree grows at high elevations limbs, this tree is often found brav- as an ornamental in Wyoming but is elevation forests of Wyoming. -

Plant Evolution and Diversity B. Importance of Plants C. Where Do Plants Fit, Evolutionarily? What Are the Defining Traits of Pl
Plant Evolution and Diversity Reading: Chap. 30 A. Plants: fundamentals I. What is a plant? What does it do? A. Basic structure and function B. Why are plants important? - Photosynthesize C. What are plants, evolutionarily? -CO2 uptake D. Problems of living on land -O2 release II. Overview of major plant taxa - Water loss A. Bryophytes (seedless, nonvascular) - Water and nutrient uptake B. Pterophytes (seedless, vascular) C. Gymnosperms (seeds, vascular) -Grow D. Angiosperms (seeds, vascular, and flowers+fruits) Where? Which directions? II. Major evolutionary trends - Reproduce A. Vascular tissue, leaves, & roots B. Fertilization without water: pollen C. Dispersal: from spores to bare seeds to seeds in fruits D. Life cycles Æ reduction of gametophyte, dominance of sporophyte Fig. 1.10, Raven et al. B. Importance of plants C. Where do plants fit, evolutionarily? 1. Food – agriculture, ecosystems 2. Habitat 3. Fuel and fiber 4. Medicines 5. Ecosystem services How are protists related to higher plants? Algae are eukaryotic photosynthetic organisms that are not plants. Relationship to the protists What are the defining traits of plants? - Multicellular, eukaryotic, photosynthetic autotrophs - Cell chemistry: - Chlorophyll a and b - Cell walls of cellulose (plus other polymers) - Starch as a storage polymer - Most similar to some Chlorophyta: Charophyceans Fig. 29.8 Points 1. Photosynthetic protists are spread throughout many groups. 2. Plants are most closely related to the green algae, in particular, to the Charophyceans. Coleochaete 3.