Prevalence of Hepatitis B in People Living
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Food Handler Exclusion Guidelines
Food Handler Exclusion Guidelines A guide for determining suitable exclusion periods for ill food handlers November 2017 Food handler exclusion guidelines Published by the State of Queensland (Queensland Health), November 2017 This document is licensed under a Creative Commons Attribution 3.0 Australia licence. To view a copy of this licence, visit creativecommons.org/licenses/by/3.0/au © State of Queensland (Queensland Health) 2017 You are free to copy, communicate and adapt the work, as long as you attribute the State of Queensland (Queensland Health). For more information contact: Food Safety Standards and Regulation, Department of Health, GPO Box 48, Brisbane QLD 4001, email [email protected], phone 07 3328 9310. An electronic version of this document is available at www.health.qld.gov.au. Disclaimer: The content presented in this publication is distributed by the Queensland Government as an information source only. The State of Queensland makes no statements, representations or warranties about the accuracy, completeness or reliability of any information contained in this publication. The State of Queensland disclaims all responsibility and all liability (including without limitation for liability in negligence) for all expenses, losses, damages and costs you might incur as a result of the information being inaccurate or incomplete in any way, and for any reason reliance was placed on such information. Food Handler Exclusion Guidelines - ii - Contents 1. Introduction ............................................................................................... -

PEDIARIX Is a Vaccine
HIGHLIGHTS OF PRESCRIBING INFORMATION • If Guillain-Barré syndrome occurs within 6 weeks of receipt of a prior These highlights do not include all the information needed to use vaccine containing tetanus toxoid, the decision to give PEDIARIX should PEDIARIX safely and effectively. See full prescribing information for be based on potential benefits and risks. (5.2) PEDIARIX. • The tip caps of the prefilled syringes contain natural rubber latex which may cause allergic reactions. (5.3) PEDIARIX [Diphtheria and Tetanus Toxoids and Acellular Pertussis • Syncope (fainting) can occur in association with administration of Adsorbed, Hepatitis B (Recombinant) and Inactivated Poliovirus injectable vaccines, including PEDIARIX. Procedures should be in place Vaccine], Suspension for Intramuscular Injection to avoid falling injury and to restore cerebral perfusion following Initial U.S. Approval: 2002 syncope. (5.4) • If temperature ≥105°F, collapse or shock-like state, or persistent, ----------------------------- INDICATIONS AND USAGE ---------------------------- PEDIARIX is a vaccine indicated for active immunization against diphtheria, inconsolable crying lasting ≥3 hours have occurred within 48 hours after tetanus, pertussis, infection caused by all known subtypes of hepatitis B virus, receipt of a pertussis-containing vaccine, or if seizures have occurred and poliomyelitis. PEDIARIX is approved for use as a 3-dose series in infants within 3 days after receipt of a pertussis-containing vaccine, the decision born of hepatitis B surface antigen (HBsAg)-negative mothers. PEDIARIX to give PEDIARIX should be based on potential benefits and risks. (5.5) may be given as early as 6 weeks of age through 6 years of age (prior to the • For children at higher risk for seizures, an antipyretic may be 7th birthday). -

Inhibition of HIV-1 by an Anti-Integrase Single-Chain Variable
Gene Therapy (1999) 6, 660–666 1999 Stockton Press All rights reserved 0969-7128/99 $12.00 http://www.stockton-press.co.uk/gt Inhibition of HIV-1 by an anti-integrase single-chain variable fragment (SFv): delivery by SV40 provides durable protection against HIV-1 and does not require selection M BouHamdan1, L-X Duan1, RJ Pomerantz1 and DS Strayer1,2 1The Dorrance H Hamilton Laboratories, Center for Human Virology, Division of Infectious Diseases, Department of Medicine; and 2Department of Pathology, Anatomy and Cell Biology, Jefferson Medical College, Thomas Jefferson University, Philadelphia, PA, USA Human immunodeficiency virus type I (HIV-1) encodes the SFv-IN was confirmed by Western blotting and several proteins that are packaged into virus particles. Inte- immunofluorescence staining, which showed that Ͼ90% of grase (IN) is an essential retroviral enzyme, which has SupT1 T-lymphocytic cells treated with SV(Aw) expressed been a target for developing agents to inhibit virus repli- the SFv-IN protein without selection. When challenged, cation. In previous studies, we showed that intracellular HIV-1 replication, as measured by HIV-1 p24 antigen expression of single-chain variable antibody fragments expression and syncytium formation, was potently inhibited (SFvs) that bind IN, delivered via retroviral expression vec- in cells expressing SV40-delivered SFv-IN. Levels of inhi- tors, provided resistance to productive HIV-1 infection in bition of HIV-1 infection achieved using this approach were T-lymphocytic cells. In the current studies, we evaluated comparable to those achieved using murine leukemia virus simian-virus 40 (SV40) as a delivery vehicle for anti-IN (MLV) as a transduction vector, the major difference being therapy of HIV-1 infection. -

Evolution of Hepatitis B Serological Markers in HIV Coinfected Patients: a Case Study
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Cadernos Espinosanos (E-Journal) Rev Saúde Pública 2017;51:24 Artigo Original http://www.rsp.fsp.usp.br/ Evolution of hepatitis B serological markers in HIV coinfected patients: a case study Ana Luiza de Castro Conde ToscanoI,II, Maria Cássia Mendes CorrêaII,III I Instituto de Infectologia Emílio Ribas. São Paulo, SP, Brasil II Departamento de Doenças Infecciosas. Faculdade de Medicina. Universidade de São Paulo. São Paulo, SP, Brasil III Instituto de Medicina Tropical de São Paulo. Laboratório de Investigação Médica 52. São Paulo, SP, Brasil ABSTRACT OBJECTIVE: To describe the evolution of serological markers among HIV and hepatitis B coinfected patients, with emphasis on evaluating the reactivation or seroreversion of these markers. METHODS: The study population consisted of patients met in an AIDS Outpatient Clinic in São Paulo State, Brazil. We included in the analysis all HIV-infected and who underwent at least two positive hepatitis B surface antigen serological testing during clinical follow up, with tests taken six months apart. Patients were tested with commercial kits available for hepatitis B serological markers by microparticle enzyme immunoassay. Clinical variables were collected: age, sex, CD4+ T-cell count, HIV viral load, alanine aminotransferase level, exposure to antiretroviral drugs including lamivudine and/or tenofovir. RESULTS: Among 2,242 HIV positive patients, we identified 105 (4.7%) patients with chronic hepatitis B. Follow up time for these patients varied from six months to 20.5 years. All patients underwent antiretroviral therapy during follow-up. Among patients with chronic hepatitis B, 58% were hepatitis B “e” antigen positive at the first assessment. -
Covalent Protein Display on Hepatitis B Core-Like Particles in Plants
www.nature.com/scientificreports OPEN Covalent protein display on Hepatitis B core‑like particles in plants through the in vivo use of the SpyTag/SpyCatcher system Hadrien Peyret1*, Daniel Ponndorf1, Yulia Meshcheriakova1, Jake Richardson2 & George P. Lomonossof1 Virus‑like particles (VLPs) can be used as nano‑carriers and antigen‑display systems in vaccine development and therapeutic applications. Conjugation of peptides or whole proteins to VLPs can be achieved using diferent methods such as the SpyTag/SpyCatcher system. Here we investigate the conjugation of tandem Hepatitis B core (tHBcAg) VLPs and the model antigen GFP in vivo in Nicotiana benthamiana. We show that tHBcAg VLPs could be successfully conjugated with GFP in the cytosol and ER without altering VLP formation or GFP fuorescence. Conjugation in the cytosol was more efcient when SpyCatcher was displayed on tHBcAg VLPs instead of being fused to GFP. This efect was even more obvious in the ER, showing that it is optimal to display SpyCatcher on the tHBcAg VLPs and SpyTag on the binding partner. To test transferability of the GFP results to other antigens, we successfully conjugated tHBcAg VLPs to the HIV capsid protein P24 in the cytosol. This work presents an efcient strategy which can lead to time and cost saving post‑translational, covalent conjugation of recombinant proteins in plants. Virus-like particles (VLPs) are protein complexes that are similar or even indistinguishable from native viral particles in terms of their structure and antigenicity. Tey do not contain the viral genome and hence, they cannot replicate and are not pathogenic. Te repetitive surface structure and a typical size range of 20–200 nm make VLPs highly immunogenic and they can induce a strong humoral and cellular immune response1. -

A Rapid Immunochromatographic Method Based on a Secondary Antibody-Labelled Magnetic Nanoprobe for the Detection of Hepatitis B Pres2 Surface Antigen
biosensors Article A Rapid Immunochromatographic Method Based on a Secondary Antibody-Labelled Magnetic Nanoprobe for the Detection of Hepatitis B preS2 Surface Antigen Yangyang Cai 1,2,3, Jun Yan 1,2,3, Li Zhu 4, Hengliang Wang 4 and Ying Lu 1,2,3,* 1 College of Food Science and Technology, Shanghai Ocean University, Shanghai 201306, China; [email protected] (Y.C.); [email protected] (J.Y.) 2 Laboratory of Quality & Safety Risk Assessment for Aquatic Products on Storage and Preservation (Shanghai), Ministry of Agriculture, Shanghai 201306, China 3 Shanghai Engineering Research Center of Aquatic-Product Processing and Preservation, Shanghai 201306, China 4 Beijing Institute of Biotechnology, Beijing 100071, China; [email protected] (L.Z.); [email protected] (H.W.) * Correspondence: [email protected]; Tel.: +86-021-6190-0503 Received: 24 September 2020; Accepted: 27 October 2020; Published: 31 October 2020 Abstract: Hepatitis B is a globally prevalent viral infectious disease caused by the hepatitis B virus (HBV). In this study, an immunochromatographic assay (ICA) for the rapid detection of hepatitis B preS2 antigen (preS2Ag) was established. The magnetic nanoparticles (MNPs) indirectly labelled with goat anti-mouse (GAM) secondary antibody were applied as a nanoprobe for free preS2 antibody (preS2Ab) capturing and signal amplification. By employing sample pre-incubation processing as well, preS2Ag-preS2Ab was sufficiently caught by the GAM-MNPs probe in 5 min. A qualitative sensitivity of 625 ng/mL was obtained by naked-eye observation within 15–20 min. A standard curve (0–5000 ng/mL) was established, with a quantitative limit of detection (LOD) of 3.6 ng/mL, based on the stability and penetrability of the magnetic signal characteristics. -
Diphtheria, Tetanus, Pertussis, Polio, Hib and Hepatitis B Vaccine for Babies and Children
Diphtheria, Tetanus, Pertussis, Polio, Hib and Hepatitis B vaccine for babies and children This leaflet tells you about the DTaP/IPV/Hib/ HepB vaccine, also known as “6 in 1” as it protects against six diseases, diphtheria, tetanus, pertussis (whooping cough), polio, Haemophilus influenzae type b and hepatitis B (HepB) disease. What does the vaccine protect against? Diphtheria Diphtheria is a serious disease that usually begins with a sore throat and can quickly cause breathing problems. It can damage the heart and nervous system and, in severe cases, can kill. Before diphtheria vaccine was introduced in the UK, there were up to 70,000 cases of diphtheria and around 5,000 deaths a year. Diphtheria can be spread from person to person through close contact. Tetanus Tetanus is a disease affecting the nervous system which can cause muscle spasms and breathing problems and can kill. It is caused when germs found in soil and manure get into the body through wounds or burns. Tetanus cannot be passed from person to person. 2 Published June 2018 Pertussis (whooping cough) Whooping cough is a disease that can cause long bouts of coughing and choking, making it hard to breathe. It can last for up to 10 weeks and babies under one year of age are most at risk. The disease is very serious and can kill. Before the pertussis vaccine was introduced, the average number of cases reported each year in the UK was 120,000 and 92 children died in the year before the vaccine was introduced. Whooping cough is usually spread by coughs and sneezes. -

Hepatitis B? HEPATITIS B Hepatitis B Is a Contagious Liver Disease That Results from Infection with the Hepatitis B Virus
What is Hepatitis B? HEPATITIS B Hepatitis B is a contagious liver disease that results from infection with the Hepatitis B virus. When first infected, a person can develop Are you at risk? an “acute” infection, which can range in severity from a very mild illness with few or no symptoms to a serious condition requiring hospitalization. Acute Hepatitis B refers to the first 6 months after someone is exposed to the Hepatitis B virus. Some people are able to fight the infection and clear the virus. For others, the infection remains and leads to a “chronic,” or lifelong, illness. Chronic Hepatitis B refers to the illness that occurs when the Hepatitis B virus remains in a person’s body. Over time, the infection can cause serious health problems. How is Hepatitis B spread? Hepatitis B is usually spread when blood, semen, or other body fluids from a person infected with the Hepatitis B virus enter the body of someone who is not infected. This can happen through having sex with an infected partner; sharing needles, syringes, or other injection drug equipment; or from direct contact with the blood or open sores of an infected person. Hepatitis B can also be passed from an infected mother to her baby at birth. Who should be tested for Hepatitis B? Approximately 1.2 million people in the United States and 350 million people worldwide have Hepatitis B. Testing for Hepatitis B is recommended for certain groups of people, including: Most are unaware of their infection. ■ People born in Asia, Africa, and other regions with moderate or high rates Is Hepatitis B common? of Hepatitis B (see map) Yes. -
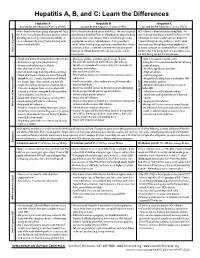
Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV. -

Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC)
AAMC Standardized Immunization Form 2020 Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC) 1. What are the hepatitis B vaccines licensed for use in the United States? Three single-antigen vaccines and two combination vaccines are currently licensed in the United States. Single-antigen hepatitis B vaccines: • ENGERIX-B® • RECOMBIVAX HB® • HEPLISAV-B™ Combination vaccines: • PEDIARIX®: Combined hepatitis B, diphtheria, tetanus, acellular pertussis (DTaP), and inactivated poliovirus (IPV) vaccine. Cannot be administered before age 6 weeks or after age 7 years. • TWINRIX®: Combined Hepatitis A and hepatitis B vaccine. Recommended for people aged ≥18 years who are at increased risk for both HAV and HBV infections. 2. What are the recommended schedules for hepatitis B vaccination? The vaccination schedule most often used for children and adults is three doses given at 0, 1, and 6 months. Alternate schedules have been approved for certain vaccines and/or populations. A new formulation, Heplisav-B (HepB-CpG), is approved to be given as two doses one month apart. 3. If there is an interruption between doses of hepatitis B vaccine, does the vaccine series need to be restarted? No. The series does not need to be restarted but the following should be considered: • If the vaccine series was interrupted after the first dose, the second dose should be administered as soon as possible. • The second and third doses should be separated by an interval of at least 8 weeks. • If only the third dose is delayed, it should be administered as soon as possible. 4. Is it harmful to administer an extra dose of hepatitis B vaccine or to repeat the entire vaccine series if documentation of the vaccination history is unavailable or the serology test is negative? No, administering extra doses of single-antigen hepatitis B vaccine is not harmful. -

Package Inserts
Individuals using assistive technology may not be able to fully access the information contained in this file. For assistance, please send an e-mail to: [email protected] and include 508 Accommodation and the title of the document in the subject line of your e-mail. HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------WARNINGS AND PRECAUTIONS------------------------ These highlights do not include all the information needed to use • Carefully consider benefits and risks before administering VAXELIS to VAXELIS safely and effectively. See full prescribing information for persons with a history of: VAXELIS. - fever ≥40.5°C (≥105°F), hypotonic-hyporesponsive episode (HHE) or VAXELISTM (Diphtheria and Tetanus Toxoids and Acellular Pertussis, persistent, inconsolable crying lasting ≥3 hours within 48 hours after a Inactivated Poliovirus, Haemophilus b Conjugate and Hepatitis B previous pertussis-containing vaccine. (5.2) Vaccine) - seizures within 3 days after a previous pertussis-containing vaccine. (5.2) Suspension for Intramuscular Injection Initial U.S. Approval: 2018 • If Guillain-Barré syndrome occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the risk for Guillain-Barré syndrome ----------------------------RECENT MAJOR CHANGES ------------------------ may be increased following VAXELIS. (5.3) Dosage and Administration (2.2) 09/2020 • Apnea following intramuscular vaccination has been observed in some ----------------------------INDICATIONS AND USAGE--------------------------- infants born prematurely. The decision about when to administer an VAXELIS is a vaccine indicated for active immunization to prevent intramuscular vaccine, including VAXELIS, to an infant born prematurely diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, and invasive disease should be based on consideration of the individual infant’s medical status due to Haemophilus influenzae type b. -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m.