Integrated Analysis of the Movement and Ecology of Wild Dingoes in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Into the Mainstream Guide to the Moving Image Recordings from the Production of Into the Mainstream by Ned Lander, 1988
Descriptive Level Finding aid LANDER_N001 Collection title Into the Mainstream Guide to the moving image recordings from the production of Into the Mainstream by Ned Lander, 1988 Prepared 2015 by LW and IE, from audition sheets by JW Last updated November 19, 2015 ACCESS Availability of copies Digital viewing copies are available. Further information is available on the 'Ordering Collection Items' web page. Alternatively, contact the Access Unit by email to arrange an appointment to view the recordings or to order copies. Restrictions on viewing The collection is open for viewing on the AIATSIS premises. AIATSIS holds viewing copies and production materials. Contact AFI Distribution for copies and usage. Contact Ned Lander and Yothu Yindi for usage of production materials. Ned Lander has donated production materials from this film to AIATSIS as a Cultural Gift under the Taxation Incentives for the Arts Scheme. Restrictions on use The collection may only be copied or published with permission from AIATSIS. SCOPE AND CONTENT NOTE Date: 1988 Extent: 102 videocassettes (Betacam SP) (approximately 35 hrs.) : sd., col. (Moving Image 10 U-Matic tapes (Kodak EB950) (approximately 10 hrs.) : sd, col. components) 6 Betamax tapes (approximately 6 hrs.) : sd, col. 9 VHS tapes (approximately 9 hrs.) : sd, col. Production history Made as a one hour television documentary, 'Into the Mainstream' follows the Aboriginal band Yothu Yindi on its journey across America in 1988 with rock groups Midnight Oil and Graffiti Man (featuring John Trudell). Yothu Yindi is famed for drawing on the song-cycles of its Arnhem Land roots to create a mix of traditional Aboriginal music and rock and roll. -

Friday Mosque Of
No.2330,Monday,17 May,2021 www.TOURISMpaper.com Some 20 historical objects have recently been confiscated from three smugglers in The World`s Only Print Tourism Newspaper Kuhdasht, western Lorestan province, a senior police official in charge of protecting cultural heritage said on Friday, CHTN reported. Including a decorative box, statue base, tray, and a few coins, the relics which had been embedded inside a car, were discovered during a routine inspection, Moham- Iranian Police madrezaMoradian announced. The official, however, did not refer to the exact age of the relics. The culprits were Seize Relics from detained and surrendered to the judicial system for further investigation, he noted. Smugglers 4 cooking Some $2m Paid to Support Tourism BozGhormeh Stew BozGhormeh Stew is a traditional recipe Businesses in South Khorasan from Kerman. Kerman is located in the he Iranian government has paid though to tempt archaeologists (rela- southeast of Iran. 1 lb (454g) Stew Beef. some 85 billion rials (over $2 tively accessible Old Esfandiar and Old Inredients: T million at the official exchange Deyhuk are amongst our favorites). ■ 1 Cup Garbanzo Beans rate of 42,000 rials per dollar) in loans Castle lovers will swoon over Birjand’s ■ 6 oz (170g) Whey Kashk to the tourism businesses affected by fortress – which might be slightly over- ■ 1 Onion the coronavirus pandemic in the eastern restored but make a great site for a tradi- ■ 2 Garlic Cloves province of South Khorasan. tional restaurant – and the mountain-top ■ 1/4 Tsp Saffron The loans have been paid to travel agen- fortifications at Qa’en, especially magi- ■ Turmeric cies, tourist complexes, and tour leaders cal at sunset; Forg is one of the most ■ Salt, White Pepper as well as handicrafts units across the picture-perfect castle-citadels in Iran. -

ORNITHOLOGIST VOLUME 44 - PARTS 1&2 - November - 2019
SOUTH AUSTRALIAN ORNITHOLOGIST VOLUME 44 - PARTS 1&2 - November - 2019 Journal of The South Australian Ornithological Association Inc. In this issue: Variation in songs of the White-eared Honeyeater Phenotypic diversity in the Copperback Quailthrush and a third subspecies Neonicotinoid insecticides Bird Report, 2011-2015: Part 1, Non-passerines President: John Gitsham The South Australian Vice-Presidents: Ornithological John Hatch, Jeff Groves Association Inc. Secretary: Kate Buckley (Birds SA) Treasurer: John Spiers FOUNDED 1899 Journal Editor: Merilyn Browne Birds SA is the trading name of The South Australian Ornithological Association Inc. Editorial Board: Merilyn Browne, Graham Carpenter, John Hatch The principal aims of the Association are to promote the study and conservation of Australian birds, to disseminate the results Manuscripts to: of research into all aspects of bird life, and [email protected] to encourage bird watching as a leisure activity. SAOA subscriptions (e-publications only): Single member $45 The South Australian Ornithologist is supplied to Family $55 all members and subscribers, and is published Student member twice a year. In addition, a quarterly Newsletter (full time Student) $10 reports on the activities of the Association, Add $20 to each subscription for printed announces its programs and includes items of copies of the Journal and The Birder (Birds SA general interest. newsletter) Journal only: Meetings are held at 7.45 pm on the last Australia $35 Friday of each month (except December when Overseas AU$35 there is no meeting) in the Charles Hawker Conference Centre, Waite Road, Urrbrae (near SAOA Memberships: the Hartley Road roundabout). Meetings SAOA c/o South Australian Museum, feature presentations on topics of ornithological North Terrace, Adelaide interest. -

The Falling Netanyahu Takes Down a Cantankerous Political Class
WWW.TEHRANTIMES.COM I N T E R N A T I O N A L D A I L Y 8 Pages Price 50,000 Rials 1.00 EURO 4.00 AED 43rd year No.13954 Saturday MAY 29, 2021 Khordad 8, 1400 Shawwal 17, 1442 Elections is Brighton’s Alireza Steel ingot export Germany recognizes competition to Jahanbakhsh to decide rises 135% in colonial-era massacres in serve people Page 2 on his future Page 3 a month on year Page 4 Namibia as genocide Page 5 Unity is the secret behind the Resistance’s victories: Amir-Abdollahian TEHRAN - Hossein Amir-Abdollahian, the resistance and steadfastness victory could special aide to the speaker of the Iranian be achieved. This victory sent an important Parliament on international affairs, has re- message that the Zionist enemy only under- flected on the secret behind the recent victory stands the language of force and resistance,” of the Palestinian resistance against Israel. he told the Lebanese Al-Ahed News. Amir-Abdollahian said the 2006 victo- The Iranian diplomat added, “This ry of the Lebanese resistance movement victory and other victories achieved by against Israel raised faith in the resistance the resistance, especially the victory of and sent a message that Israel understands July 2006, were achieved in light of the only the language of power. unity of the Lebanese people and the gold- “The flight of the Zionist entity from en equation in Lebanon – the army, the southern Lebanon raised faith in the resist- people, and the resistance. Crabs in ance among the Lebanese, and that through Continued on page 3 Tehran, Moscow confer on joint investment in agricultural sector TEHRAN – Iranian Agriculture Minis- investment in various agricultural fields. -

Black North American and Caribbean Music in European Metropolises a Transnational Perspective of Paris and London Music Scenes (1920S-1950S)
Black North American and Caribbean Music in European Metropolises A Transnational Perspective of Paris and London Music Scenes (1920s-1950s) Veronica Chincoli Thesis submitted for assessment with a view to obtaining the degree of Doctor of History and Civilization of the European University Institute Florence, 15 April 2019 European University Institute Department of History and Civilization Black North American and Caribbean Music in European Metropolises A Transnational Perspective of Paris and London Music Scenes (1920s- 1950s) Veronica Chincoli Thesis submitted for assessment with a view to obtaining the degree of Doctor of History and Civilization of the European University Institute Examining Board Professor Stéphane Van Damme, European University Institute Professor Laura Downs, European University Institute Professor Catherine Tackley, University of Liverpool Professor Pap Ndiaye, SciencesPo © Veronica Chincoli, 2019 No part of this thesis may be copied, reproduced or transmitted without prior permission of the author Researcher declaration to accompany the submission of written work Department of History and Civilization - Doctoral Programme I Veronica Chincoli certify that I am the author of the work “Black North American and Caribbean Music in European Metropolises: A Transnatioanl Perspective of Paris and London Music Scenes (1920s-1950s). I have presented for examination for the Ph.D. at the European University Institute. I also certify that this is solely my own original work, other than where I have clearly indicated, in this declaration and in the thesis, that it is the work of others. I warrant that I have obtained all the permissions required for using any material from other copyrighted publications. I certify that this work complies with the Code of Ethics in Academic Research issued by the European University Institute (IUE 332/2/10 (CA 297). -

Miles Davis, Tutu
Gilbert DUPUIS Guitariste, titulaire du D.E.de jazz, professeur certifié d’éducation musicale Miles Davis, Tutu Inscrire Miles Davis au programme du baccalauréat est une façon officielle de rendre enfin hommage à une figure légendaire du jazz, peut-être la plus haute, et, de surcroît, à l’une des plus grandes stars de la musique afro-américaine. Avec cet album, il s’impose également d’évoquer les nouvelles technologies et la musique programmée, singularité dans l’œuvre du trompettiste. Cet article propose une vaste étude de la musique de Miles Davis et de ses contemporains. Les principaux courants sont présentés depuis le be-bop jusqu’au doo-bop, via la période électrique. Suit un avant-propos de l’ensemble de l’album Tutu, avant de se concentrer dans le détail à l’analyse musicale des trois morceaux imposés (Tutu, Tomaas, Portia). Préambule peintre (au sens propre comme au sens figuré), « coloriste », « architecte » des sons, « sculpteur », e « L’histoire du jazz au 20 siècle est aussi variée, « conteur », « poète » de la trompette, « Miles » s’est complexe, voire profuse, que la musique dite essayé, on ne peut dire à tous les courants, mais à sérieuse ; des liens qui les unissent existent pourtant, la plupart des styles ouvrant à la modernité qu’il souvent plus resserrés, intenses et intimes qu’on a très fréquemment impulsés. C’est en ce sens, 1 pourrait le suspecter . » probablement, le musicien le plus évolutif, celui Cette phrase semble vraie. Mais, comme d’autres qui ne regarde pas derrière lui et ne s’arrête pas en sur ce sujet, elle n’est pas ou plus tout à fait chemin, sauf pour laisser place au silence, oubliant juste. -

On Track 2010-2011
ON TRACK Delivering NRM in the SA Arid Lands 2010-11 ON TRACK Delivering natural resources management in the SA Arid Lands 2010-11 Protecting our land, plants and animals Understanding and securing our water resources Supporting our industries and communities 1 Welcome It is with great pleasure that I introduce this first edition of On Track. Having now completed the first year the achievements of former Presiding of delivery of the South Australian Arid Member Chris Reed, previous members Lands (SAAL) Regional Natural Resources of the Board, and General Manager John Management (NRM) Plan which sets the Gavin. Almost all of the activities you will direction for natural resources management read about here were initiated through their in the region to 2020, On Track is a report efforts and the current Board is building on to our community on the progress we made their endeavours. in 2010-11 on meeting the Plan’s targets. This year was also marked by the True to the SAAL NRM Board’s platform establishment of the new Department of and the spirit of natural resources Environment and Natural Resources in July management, On Track’s focus is on 2010 which brings together staff from the community. Outback office of the former Department We showcase the variety of projects and for Environment and Heritage and the staff activities where community members are of the SAAL NRM Board. working with the Board. This new integrated service will use a We share with you the experiences of landscape approach to manage natural some of the landholders and community resources across public and private land members involved with our programs and provide a single face for environment including Ecosystem Management and natural resources services in our Understanding™, Pest Management and region. -

Born in America, Jazz Can Be Seen As a Reflection of the Cultural Diversity and Individualism of This Country
1 www.onlineeducation.bharatsevaksamaj.net www.bssskillmission.in “Styles in Jazz Music”. In Section 1 of this course you will cover these topics: Introduction What Is Jazz? Appreciating Jazz Improvisation The Origins Of Jazz Topic : Introduction Topic Objective: At the end of this topic student would be able to: Discuss the Birth of Jazz Discuss the concept of Louis Armstrong Discuss the Expansion of Jazz Understand the concepts of Bebop Discuss todays Jazz Definition/Overview: The topic discusses that the style of music known as jazz is largely based on improvisation. It has evolved while balancing traditional forces with the pursuit of new ideas and approaches. Today jazz continues to expand at an exciting rate while following a similar path. Here you will find resources that shed light on the basics of one of the greatest musical developments in modern history.WWW.BSSVE.IN Born in America, jazz can be seen as a reflection of the cultural diversity and individualism of this country. At its core are openness to all influences, and personal expression through improvisation. Throughout its history, jazz has straddled the worlds of popular music and art music, and it has expanded to a point where its styles are so varied that one may sound completely unrelated to another. First performed in bars, jazz can now be heard in clubs, concert halls, universities, and large festivals all over the world. www.bsscommunitycollege.in www.bssnewgeneration.in www.bsslifeskillscollege.in 2 www.onlineeducation.bharatsevaksamaj.net www.bssskillmission.in Key Points: 1. The Birth of Jazz New Orleans, Louisiana around the turn of the 20th century was a melting pot of cultures. -

National Recovery Plan for the Plains Mouse Pseudomys Australis 2012
National Recovery Plan for the Plains Mouse Pseudomys australis 2012 - 1 - This plan should be cited as follows: Moseby, K. (2012) National Recovery Plan for the Plains Mouse Pseudomys australis. Department of Environment, Water and Natural Resources, South Australia. Published by the Department of Environment, Water and Natural Resources, South Australia. Adopted under the Environment Protection and Biodiversity Conservation Act 1999: [date to be supplied] ISBN : 978-0-9806503-1-0 © Department of Environment, Water and Natural Resources, South Australia. This publication is copyright. Apart from any use permitted under the copyright Act 1968, no part may be reproduced by any process without prior written permission from the Government of South Australia. Requests and inquiries regarding reproduction should be addressed to: Department of Environment, Water and Natural Resources GPO Box 1047 ADELAIDE SA 5001 Note: This recovery plan sets out the actions necessary to stop the decline of, and support the recovery of, the listed threatened species or ecological community. The Australian Government is committed to acting in accordance with the plan and to implementing the plan as it applies to Commonwealth areas. The plan has been developed with the involvement and cooperation of a broad range of stakeholders, but individual stakeholders have not necessarily committed to undertaking specific actions. The attainment of objectives and the provision of funds may be subject to budgetary and other constraints affecting the parties involved. Proposed actions may be subject to modification over the life of the plan due to changes in knowledge. Queensland disclaimer: The Australian Government, in partnership with the Queensland Department of Environment and Heritage Protection, facilitates the publication of recovery plans to detail the actions needed for the conservation of threatened native wildlife. -

UNDERSTANDING MILES DAVIS in 9 PARTS by Greg Cwik and David Marchese ______
UNDERSTANDING MILES DAVIS IN 9 PARTS by Greg Cwik and David Marchese ________________________________________________________ [*This article appeared in the September 21, 2015 issue of New York Magazine.] PHOTO CREDIT DAVID REDFERN/REDFERNS/GETTY IMAGES hen Miles Ahead, directed by and starring Don Cheadle, premieres at the New York Film Festival in October, 2015, it’ll mark the first time that the W jazz genius Miles Davis has been the subject of a non-documentary film. It wasn’t a lack of interest that has kept Davis from movie theaters till now. Nor was it lack of material: Davis, who died in 1991, lived a dynamic and controversial life, both personally and musically. (Multiple biopics wound up in development hell.) Cheadle’s film focuses on a period when the trumpeter was living in seclusion, so here’s a broader overview of one of American music’s true giants. 1. What to Know About His Sound ____________________________________________ Grammy-nominated trumpeter and jazz educator Jon Faddis explains: “The thing that is special about his sound is economy when he was improvising. Before Miles, most people thought of the trumpet as a very extroverted instrument. Miles was more introverted in his approach. He used a Harmon mute — it wasn’t really popular before he started to use it — and it’s a very beautiful sound. I also 1 Jon Faddis: one of the things that set Miles apart was his use of space, making the space a part of the music… think his minimal use of vibrato was a tremendous influence on instrumentalists. Recordings like Round Midnight, Someday My Prince Will Come, I Thought About You were ingenious. -
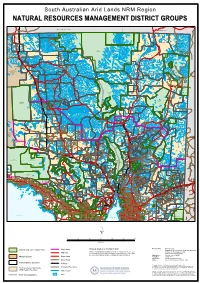
Natural Resources Management District Groups
South Australian Arid Lands NRM Region NNAATTUURRAALL RREESSOOUURRCCEESS MMAANNAAGGEEMMEENNTT DDIISSTTRRIICCTT GGRROOUUPPSS NORTHERN TERRITORY QUEENSLAND Mount Dare H.S. CROWN POINT Pandie Pandie HS AYERS SIMPSON DESERT RANGE SOUTH Tieyon H.S. CONSERVATION PARK ALTON DOWNS TIEYON WITJIRA NATIONAL PARK PANDIE PANDIE CORDILLO DOWNS HAMILTON DEROSE HILL Hamilton H.S. SIMPSON DESERT KENMORE REGIONAL RESERVE Cordillo Downs HS PARK Lambina H.S. Mount Sarah H.S. MOUNT Granite Downs H.S. SARAH Indulkana LAMBINA Todmorden H.S. MACUMBA CLIFTON HILLS GRANITE DOWNS TODMORDEN COONGIE LAKES Marla NATIONAL PARK Mintabie EVERARD PARK Welbourn Hill H.S. WELBOURN HILL Marla - Oodnadatta INNAMINCKA ANANGU COWARIE REGIONAL PITJANTJATJARAKU Oodnadatta RESERVE ABORIGINAL LAND ALLANDALE Marree - Innamincka Wintinna HS WINTINNA KALAMURINA Innamincka ARCKARINGA Algebuckinna Arckaringa HS MUNGERANIE EVELYN Mungeranie HS DOWNS GIDGEALPA THE PEAKE Moomba Evelyn Downs HS Mount Barry HS MOUNT BARRY Mulka HS NILPINNA MULKA LAKE EYRE NATIONAL MOUNT WILLOUGHBY Nilpinna HS PARK MERTY MERTY Etadunna HS STRZELECKI ELLIOT PRICE REGIONAL CONSERVATION ETADUNNA TALLARINGA PARK RESERVE CONSERVATION Mount Clarence HS PARK COOBER PEDY COMMONAGE William Creek BOLLARDS LAGOON Coober Pedy ANNA CREEK Dulkaninna HS MABEL CREEK DULKANINNA MOUNT CLARENCE Lindon HS Muloorina HS LINDON MULOORINA CLAYTON Curdimurka MURNPEOWIE INGOMAR FINNISS STUARTS CREEK SPRINGS MARREE ABORIGINAL Ingomar HS LAND CALLANNA Marree MUNDOWDNA LAKE CALLABONNA COMMONWEALTH HILL FOSSIL MCDOUAL RESERVE PEAK Mobella -

M01: Mineral Exploration Licence Applications
M01 Mineral Exploration Licence Applications 27 September 2021 Resources and Energy Group L4 11 Waymouth Street, Adelaide SA 5000 http://energymining.sa.gov.au/minerals GPO Box 320, ADELAIDE, SA 5001 http://energymining.sa.gov.au/energy_resources Phone +61 8 8463 3000 http://map.sarig.sa.gov.au Email [email protected] Earth Resources Information Sheet - M1 Printed on: 27/09/2021 M01: Mineral Exploration Licence Applications Year Lodged: 1996 File Ref. Applicant Locality Fee Zone Area (km2 ) 250K Map 1996/00118 NiCul Minerals Limited Mount Harcus area - Approx 400km 2,415 Lindsay, WNW of Marla Woodroffe 1996/00185 NiCul Minerals Limited Willugudinna Hill area - Approx 823 Everard 50km NW of Marla 1996/00260 Goldsearch Ltd Ernabella South area - Approx 519 Alberga 180km NW of Marla 1996/00262 Goldsearch Ltd Marble Hill area - Approx 80km NW 463 Abminga, of Marla Alberga 1996/00340 Goldsearch Ltd Birksgate Range area - Approx 2,198 Birksgate 380km W of Marla 1996/00341 Goldsearch Ltd Ayliffe Hill area - Approx 220km NW 1,230 Woodroffe of Marla 1996/00342 Goldsearch Ltd Musgrave Ranges area - Approx 2,136 Alberga 180km NW of Marla 1996/00534 Caytale Pty Ltd Bull Hill area - Approx 240km NW of 1,783 Woodroffe Marla Year Lodged: 1997 File Ref. Applicant Locality Fee Zone Area (km2 ) 250K Map 1997/00040 Minex (Aust) Pty Ltd Bowden Hill area - Approx 300 WNW 1,507 Woodroffe of Marla 1997/00053 Mithril Resources Limited Mt Cooperina Area - approx. 360 km 1,013 Mann WNW of Marla 1997/00055 Mithril Resources Limited Oonmooninna Hill Area - approx.