2015 Matchayaw Lake Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sturgeon River Watershed Management Plan
2020 Sturgeon River Watershed Management Plan PREPARED BY ENDORSEMENTS The following municipalities accept the Sturgeon River Watershed Management Plan (2020) as information and resolve to: 1. continue to work collaboratively with other municipalities and the Sturgeon River Watershed Alliance to implement the plan; and 2. to consider plan recommendations in the decision-making of the municipality including the development of new or updated statutory and other documents. Municipality Signature Position Date City of Edmonton City of Spruce Grove City of St. Albert Lac Ste. Anne County Parkland County Sturgeon County Village of Alberta Beach Summer Villages of Lac Ste Anne County East (SVLSACE) Town of Gibbons Town of Morinville Town of Onoway Town of Stony Plain 2 Additionally, the Sturgeon River Watershed Management Plan (2020) has been endorsed by the following: Organization Signature Position Date Alberta Conservation Association Big Lake Environmental Support Society North Saskatchewan Watershed Alliance Sturgeon River Watershed Alliance Wagner Natural Area Society 3 EXECUTIVE SUMMARY In support of Water for Life: Alberta’s strategy for sustainability, the Sturgeon River Watershed Alliance (SRWA) is a watershed stewardship group made up of communities and organizations operating in the Sturgeon River watershed. The vision of the SRWA is: The Sturgeon River watershed is recognized and valued as a natural prairie river system that connects our communities and adds to our quality of life. It is healthy, sustaining its ecological structure, processes, functions, and resiliency, within its range of natural variability. It is collaboratively stewarded by rural landowners, urban residents and Indigenous communities, and is managed with knowledge and ecological integrity as the foundation for decision-making that balances our social, cultural, economic and environmental well-being. -

Jackfish Lake State of the Watershed Report
Jackfish Lake State of the Watershed Report April 2016 North Saskatchewan Watershed Alliance 202 - 9440 49th St NW Edmonton, AB T6B 2M9 Tel: (587) 525-6820 Email: [email protected] http://www.nswa.ab.ca The North Saskatchewan Watershed Alliance (NSWA) is a non-profit society whose purpose is to protect and improve water quality and ecosystem functioning in the North Saskatchewan River watershed in Alberta. The organization is guided by a Board of Directors composed of member organizations from within the watershed. It is the designated Watershed Planning and Advisory Council (WPAC) for the North Saskatchewan River under the Government of Alberta’s Water for Life Strategy.. This report was prepared by Jennifer Regier, B.Sc. and David Trew, P.Biol of the North Saskatchewan Watershed Alliance. Cover photo credit: Dara Choy, Stony Plain AB Suggested Citation: North Saskatchewan Watershed Alliance (NSWA). 2016. Jackfish Lake State of the Watershed Report. Prepared by the NSWA, Edmonton, AB. for the Jackfish Lake Management Association, Carvel, AB. Available on the internet at http://www.nswa.ab.ca/resources/ nswa_publications Jackfish Lake State of the Watershed Report Executive Summary The purpose of this report is to consolidate environmental information on Jackfish Lake and its watershed in an effort to support future planning and management discussions. The report provides perspective on current environmental conditions at the lake relative to regional and historic trends. The report is provided as advice to the Jackfish Lake Management Association (JLMA), Alberta Environment and Parks, and Parkland County. The technical information contained in this document is detailed and addresses many lake and watershed features. -

Wabamun Lake Water Quality 1982 to 2001
WABAMUN LAKE WATER QUALITY 1982 TO 2001 WABAMUN LAKE WATER QUALITY 1982 TO 2001 Prepared by: Richard Casey, M.Sc. Limnologist Science and Standards Alberta Environment September 2003 W0309 Pub. No: T/695 ISBN: 0-7785-2503-1 (Printed Edition) ISBN: 0-7785-2504-X (On-Line Edition) Web Site: http://www3.gov.ab.ca/env/info/infocentre/publist.cfm Any comments, questions, or suggestions regarding the content of this document may be directed to: Environmental Monitoring and Evaluation Branch Alberta Environment 10th Floor, Oxbridge Place 9820 – 106th Street Edmonton, Alberta T5K 2J6 Phone: (780) 427-6278 Fax: (780) 422-6712 Additional copies of this document may be obtained by contacting: Information Centre Alberta Environment Main Floor, Great West Life Building 9920 – 108th Street Edmonton, Alberta T5K 2M4 Phone: (780) 944-0313 Fax: (780) 427-4407 Email: [email protected] SUMMARY Wabamun Lake, approximately 60 km west of Edmonton, is large, shallow, and generally well mixed. Sport fish in the lake include northern pike, yellow perch, and lake whitefish. There are a unique mix of land uses in the lake watershed, which include undisturbed bush and forest, agriculture, two coal mines with active and reclaimed areas, three coal-fired power plants, major transportation (road and rail) corridors, residences, and recreation. The mines supply fuel for the power plants, operated by the TransAlta Utilities Corporation (TAU). Industrial wastewaters, runoff and cooling water from the Whitewood mine and Wabamun power plant are discharged to the lake. Over time, TAU operations associated with the mines and power plants in the watershed have caused cumulative and ongoing impacts on the lake level. -

The Town of Onoway
Welcome to The Town of Onoway Situated in the scenic Sturgeon River valley, the Town of Onoway, Alberta with a population of 1,029, is located on gently rolling farmland in the southeast corner of Lac Ste. Anne County. Onoway provides a small-town country lifestyle, along with easy access to major urban centres. The town is well positioned at the junction of Highways 43 and 37 and is approximately 50 km directly northwest of Edmonton and 35 km northwest of Spruce Grove. Being in the proximity of the outer commuter zone for the greater Edmonton metro region allows people to live in Onoway and enjoy the more affordable and quieter country lifestyle while working elsewhere. Likewise, the Town’s proximity to the two highways also allows people to live elsewhere while being employed in Onoway. The greater connectivity of Onoway with Stony Plain, Spruce Grove, St. Albert and Edmonton has been good for the Town. It gives residents more options for work and recreation, and businesses have a greater potential market. The community has deep roots as an agricultural community going back at least 100 years. The Town of Onoway, benefits from a local trading area of more than 16,000 with a large number of country residential subdivisions and summer villages in the area supporting its retail businesses and professional service sectors. Onoway has become a small hub for the East Lac Ste. Anne region, providing vital education, health, retail, recreational and social services to residents of the town and surrounding rural areas. Topography Onoway is surrounded by an interesting landscape which is characterized by moderately rolling, hilly topography and big bodies of water. -

An Ecological Study of Wildlife and Fisheries in the Pembina and Sturgeon River Basins
AN ECOLOGICAL STUDY OF WILDLIFE AND FISHERIES IN THE PEMBINA AND STURGEON RIVER BASINS — Volume 1 — RESULTS OF ECOLOGICAL STUDIES < QL PREPARED FOR 84.26 A2 Water Resources Division SUIcWCE LIBRARY El 9 ALBERTA DEPARTMENT OF THE ENVIRO NM ENT , 1971 v . 1 ENEWABLB RESOURCES CONSULTING SERVICES LTD. SCI NOVEMBER, 1971 iv+iiy TABLE OF CONTENTS page I. Introduction .......................................... 1 II. The Study Area ........................................ 3 III. Wildlife Study A. Wildlife Habitat ................................... 6 1. Terrestrial Vegetation a. Methods ...................................... 7 b. Results - Extent of Forest Types along the Sturgeon and Pembina Rivers 1. Sturgeon River ........................... 9 2. Pembina River ............................ 19 Composition of Forest Associations Studied in the Sturgeon and Pembina River Basins ... 22 c. Summary ...................................... 44 2. Aquatic Vegetation a. Methods ...................................... 50 b. Results 1. Lakes ...........'.......................... 51 Summary ................................ 64 2. Rivers Sturgeon River ......................... 66 Pembina River .......................... 72 B. Wildlife Populations 1. Waterfowl in the Sturgeon and Pembina River Basins . ......................................... 75 a. Breeding Pair Survey 1. Methods ................................... 75 2. Results and Discussion .................. 77 Lakes ................................... 7 8 Sturgeon River ......................... 92 Pembina -
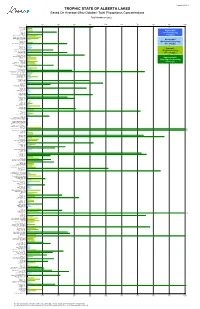
Trophic State of Alberta Lakes Based on Average Total Phosphorus
Created Feb 2013 TROPHIC STATE OF ALBERTA LAKES Based On Average (May-October) Total Phosphorus Concentrations Total Phosphorus (µg/L) 0 100 200 300 400 500 600 700 800 900 1000 * Adamson Lake Alix Lake * Amisk Lake * Angling Lake Oligotrophic * ‡ Antler Lake Arm Lake (Low Productivity) * Astotin Lake (<10 µg/L) * ‡ Athabasca (Lake) - Off Delta Baptiste Lake - North Basin Baptiste Lake - South Basin * ‡ Bare Creek Res. Mesotrophic * ‡ Barrier Lake ‡ Battle Lake (Moderate Productivity) * † Battle River Res. (Forestburg) (10 - 35 µg/L) Beartrap Lake Beauvais Lake Beaver Lake * Bellevue Lake Eutrophic * † Big Lake - East Basin * † Big Lake - West Basin (High Productivity) * Blackfalds Lake (35 - 100 µg/L) * † Blackmud Lake * ‡ Blood Indian Res. Bluet (South Garnier Lake) ‡ Bonnie Lake Hypereutrophic † Borden Lake * ‡ Bourque Lake (Very High Productivity) ‡ Buck Lake (>100 µg/L) Buffalo Lake - Main Basin Buffalo Lake - Secondary Bay * † Buffalo Lake (By Boyle) † Burntstick Lake Calling Lake * † Capt Eyre Lake † Cardinal Lake * ‡ Carolside Res. - Berry Creek Res. † Chain Lakes Res. - North Basin † Chain Lakes Res.- South Basin Chestermere Lake * † Chickakoo Lake * † Chickenhill Lake * Chin Coulee Res. * Clairmont Lake Clear (Barns) Lake Clear Lake ‡ Coal Lake * ‡ Cold Lake - English Bay ‡ Cold Lake - West Side ‡ Cooking Lake † Cow Lake * Crawling Valley Res. Crimson Lake Crowsnest Lake * † Cutbank Lake Dillberry Lake * Driedmeat Lake ‡ Eagle Lake ‡ Elbow Lake Elkwater Lake Ethel Lake * Fawcett Lake * † Fickle Lake * † Figure Eight Lake * Fishing Lake * Flyingshot Lake * Fork Lake * ‡ Fox Lake Res. Frog Lake † Garner Lake Garnier Lake (North) * George Lake * † Ghost Res. - Inside Bay * † Ghost Res. - Inside Breakwater ‡ Ghost Res. - Near Cochrane * Gleniffer Lake (Dickson Res.) * † Glenmore Res. -

Mercury in Fish 2009-2013
2016 Mercury in Fish In Alberta Water Bodies 2009–2013 For more information on Fish Consumption Advisories Contact: Health Protection Branch Alberta Health P.O. Box 1360, Station Main Edmonton, Alberta, T5J 1S6 Telephone: 1-780-427-1470 ISBN: 978-0-7785- 8283-0 (Report) ISBN: 978-0-7785- 8284-7 (PDF) 2016 Government of Alberta Alberta Health, Health Protection Branch Mercury in Fish in Alberta Water Bodies 2009 – 2013 February 2016 Executive Summary Mercury enters the environment through various natural processes and human activities. Methylmercury is transformed from inorganic forms of mercury via methylation by micro-organisms in natural waters, and can accumulate in some fish. Humans are exposed to very low levels of mercury directly from the air, water and food. Fish consumers may be exposed to relatively higher levels of methylmercury by eating mercury-containing fish from local rivers and lakes. Methylmercury can accumulate in the human body over time. Because methylmercury is a known neurotoxin, it is necessary to limit human exposure. From 2009 to 2013, the Departments of Environment and Parks (AEP) and Health (AH) initiated a survey of mercury levels in fish in selected water bodies in Alberta. These water bodies are extensively accessed by the public for recreational activities. This report deals with (1) concentrations of total mercury levels in various fish species collected from the water bodies in Alberta, (2) estimated exposures, (3) fish consumption limits, (4) fish consumption advisories, and (5) health benefits of fish consumption. The results indicate that: 1. Concentrations of total mercury in fish in the water bodies in Alberta were within the ranges for the same fish species from other water bodies elsewhere in Canada and the United States. -

PP2 - Lakes, Reservoirs and Ponds Waterbody Waterbody Detail Season Bait WALL NRPK YLPR LKWH BURB Trout Total L = Bait Allowed Arm Lake OPEN MAY 15 to MAR
Legend: As examples, ‘3 over 63 cm’ indicates a possession and size limit of ‘3 fish each over 63 cm’ or ‘10 fish’ indicates a possession limit of 10 for that species of any size. An empty cell indicates the species is not likely present at that waterbody; however, if caught the default regulations for the Watershed Unit apply. SHL=Special Harvest Licence, BKTR = Brook Trout, BNTR=Brown Trout, BURB = Burbot, CISC = Cisco, CTTR = Cutthroat Trout, DLVR = Dolly Varden, GOLD = Goldeye, LKTR = Lake Trout, LKWH = Lake Whitefish, MNWH = Mountain Whitefish, NRPK = Northern Pike, RNTR = Rainbow Trout, SAUG = Sauger, TGTR = Tiger Trout, WALL = Walleye, YLPR = Yellow Perch. Regulation changes are highlighted blue. Waterbodies closed to angling are highlighted grey. PP2 - Lakes, Reservoirs and Ponds Waterbody Waterbody Detail Season Bait WALL NRPK YLPR LKWH BURB Trout Total l = Bait allowed Arm Lake OPEN MAY 15 TO MAR. 31 l 3 over 15 fish 63 cm Battle Lake Portion west of the west boundary of section 22-46-2-W5; OPEN JUNE 1 TO MAR. 31 l 0 fish 0 fish 5 fish 10 fish 2 fish; but limit Includes tributaries and outlet downstream to Sec. Rd. 771 is 0 from Feb. 1 to Mar. 31 Remainder of the lake OPEN MAY 15 TO MAR. 31 l 0 fish 0 fish 5 fish 10 fish 2 fish; but limit is 0 from Feb. 1 to Mar. 31 Berry Creek (Carolside) Reservoir 27-12-W4 OPEN MAY 15 TO MAR. 31 l 1 fish 45-50 1 fish 15 fish cm 63-70 cm Big Lake Includes tributaries OPEN MAY 15 TO MAR. -

Environmental Baseline Studies. Fish and Aquatic Resources
Environmental Baseline Study: Fish and Aquatic Resources Total E&P Canada Ltd. Calgary, Alberta BBinderinder SSectionection PPages.inddages.indd ccover1over1 112/11/20072/11/2007 11:52:34:52:34 PPMM Environmental Baseline Study: Fish and Aquatic Resources Table of Contents Table of Contents 1 Introduction ................................................................................................................................ 1-1 1.1 Background .................................................................................................................................. 1-1 1.2 Focus of Baseline Investigations .................................................................................................. 1-1 1.3 Study Areas .................................................................................................................................. 1-1 1.3.1 Local Study Area ............................................................................................................. 1-1 1.3.2 Regional Study Area ....................................................................................................... 1-3 2 Methods ....................................................................................................................................... 2-1 2.1 Historical Data .............................................................................................................................. 2-1 2.2 Data Collection ............................................................................................................................ -

Infrastructuremasterplan Sturge
Corporate Authorization This document entitled “Sturgeon County Infrastructure Master Plan” has been prepared by ISL Engineering and Land Services Ltd. (ISL) for the use of Sturgeon County. The information and data provided herein represent ISL’s professional judgment at the time of preparation. ISL denies any liability whatsoever to any other parties who may obtain this report and use it, or any of its contents, without prior written consent from ISL. Barry Raynard, M.Eng., P.Eng. Daniel Zeggelaar, P.Eng., PTOE Manager, Municipal/Water Resources Engineering Project Manager – Transportation islengineering.com Sturgeon County Infrastructure Master Plan July 2019 Sturgeon County – Report i FINAL Council endorsed Sturgeon County's Infrastructure Master Plan to be used as a key infrastructure planning input for the community. Motion 338/19 September 10th, 2019 Table of Contents 1.0 Introduction .................................................................................................................. 1 1.1 Background 2 1.2 Study Purpose 2 1.3 Terminology 2 2.0 Methodology ................................................................................................................ 4 2.1 Project Management Approach 5 2.2 Data Collection 5 2.3 Trends / Growth Projections 5 2.4 Existing Infrastructure Analysis 5 2.5 Future Infrastructure Analysis 6 2.6 Implementation Strategy 6 2.7 Performance Indicators 7 3.0 Trends and Growth Projections .................................................................................... 8 3.1 Introduction -

Water and Energy in the North Saskatchewan River Watershed
IntegratedIntegrated WatershedWatershed ManagementManagement PlanningPlanning forfor thethe NorthNorth SaskatchewanSaskatchewan RiverRiver BasinBasin Presented to AlbertaAlberta HealthHealth SeptSept 17,17, 20092009 OutlineOutline WaterWater forfor LifeLife (2003)(2003) NSWANSWA functionfunction WatershedWatershed PlanningPlanning InitiativesInitiatives LakeLake ManagementManagement ChallengesChallenges WaterWater forfor LifeLife (2003)(2003) OutcomesOutcomes:: ¾ SafeSafe drinkingdrinking waterwater ¾ HealthyHealthy aquaticaquatic ecosystemsecosystems ¾ ReliableReliable waterwater suppliessupplies KeyKey DirectionsDirections:: ¾ KnowledgeKnowledge andand researchresearch ¾ WaterWater conservationconservation ¾ PartnershipsPartnerships forfor watershedwatershed managementmanagement andand stewardshipstewardship WaterWater forfor LifeLife RenewalRenewal (2008)(2008) Nov.Nov. 16,16, 20082008 MinsterMinster RennerRenner confirmsconfirms originaloriginal WFLWFL outcomesoutcomes andand keykey directionsdirections WPACWPAC rolerole inin watershedwatershed assessmentassessment andand planningplanning rere-- confirmedconfirmed WatershedWatershed PlanningPlanning AdvisoryAdvisory CouncilsCouncils NineNine WPACsWPACs havehave beenbeen Hay River watershed appointedappointed inin AlbertaAlberta • MandateMandate:: Peace River Watershed Athabasca River ¾¾StateState ofof thethe Watershed WatershedWatershed ReportsReports Lesser Slave Lake Watershed Council Beaver River ¾¾IntegratedIntegrated WatershedWatershed Watershed Alliance North Saskatchewan -

Sport Fisheries Restoration at Isle and Hasse Lakes
Alberta Conservation Association 2015/16 Project Summary Report Project Name: Sport Fisheries Restoration at Isle and Hasse Lakes Fisheries Program Manager: Peter Aku Project Leader: Brendan Ganton Primary ACA staff on project: Troy Furukawa, Brendan Ganton, Britt Keeling, Scott Seward and Juanna Thompson Partnerships Environment Canada – Environmental Damages Fund Lake Isle & Lac Ste. Anne Water Quality Management Society North Saskatchewan Watershed Alliance Parkland County and Lac Ste. Anne County Alternative Land Use Services Program Sturgeon River Watershed Alliance Key Findings • We established partnerships with a variety of stakeholders interested in fisheries restoration at both Isle and Hasse lakes, including Parkland County, Lac Ste. Anne County and the Sturgeon River Watershed Alliance. • Levels of chlorophyll-a, phosphorus, E. coli and fecal coliforms were low at Hasse Lake. • The fish community at Hasse Lake consisted predominantly of fathead minnows, with a few brook stickleback; no large-bodied fish species were recorded. • Mature northern pike, walleye and yellow perch were found in Isle Lake despite previous concerns of complete winterkill, suggesting some fish may have survived the winterkill events or migrated from nearby waterbodies. Introduction Eutrophication and deteriorating water quality of lakes are common problems in developed areas of Alberta. In recent decades, changes in watershed land use have resulted in increased nutrient runoff, particularly phosphorus, into many lakes, such as Isle and Hasse lakes. Increased phosphorus to aquatic systems can result in substantial increases in phytoplankton production and algal blooms, including blue-green cyanobacteria blooms. Aside from aesthetic concerns, algal blooms have been linked with anoxic water conditions and toxic or harmful effects on fisheries, human health and recreation.