Morphological Studies on the Last Instar Larva of Ampulex Compressa (Fabricius) from Brazil
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Halloween Horrors: the Dark Side of Mother Nature by Francie Mcgowan
Halloween Horrors: The Dark Side of Mother Nature by Francie McGowan As Halloween approaches, monsters, bats and bugs will loom in the darkness of a moonless night in October to scare us. Mother Nature also has some macabre critters of the bug and insect variety that are every bit as eerie and unsettling as any Halloween costume or horror film. [Spoiler alert: do no read this article while eating or you may get sick.] To start with a seemingly pious bug, the praying mantis (order Mantodea), is actually a deadly eater and mater. With her long forelegs she captures her prey and eats them alive while holding them in a death grip. While she is mating, she bites the head of the male clean off and continues chomping the rest of the poor victim until he dies. She then saunters off well fed and fertile. The Japanese giant hornet (Vespa mandarinia japonica) is so big that, in flight, it resembles a small bird. It stings or sprays its victims - including humans - with a flesh-dissolving acid. It usually aims for the eyes. Embedded in this acid is a pheromone that attracts the other hornets in the hive to the victim and they attack en masse. Thirty of these hornets can attack a honey bee hive and kill thirty thousand of them in a matter of a few hours. Another creature to throw the most stalwart person into a state of arachnophobia (fear of spiders) or entomophobia (fear of insects) is the cockroach wasp (Ampulex compressa). They live in Asia and in Africa. -

Arquivos De Zoologia MUSEU DE ZOOLOGIA DA UNIVERSIDADE DE SÃO PAULO
Arquivos de Zoologia MUSEU DE ZOOLOGIA DA UNIVERSIDADE DE SÃO PAULO ISSN 0066-7870 ARQ. ZOOL. S. PAULO 37(1):1-139 12.11.2002 A SYNONYMIC CATALOG OF THE NEOTROPICAL CRABRONIDAE AND SPHECIDAE (HYMENOPTERA: APOIDEA) SÉRVIO TÚLIO P. A MARANTE Abstract A synonymyc catalogue for the species of Neotropical Crabronidae and Sphecidae is presented, including all synonyms, geographical distribution and pertinent references. The catalogue includes 152 genera and 1834 species (1640 spp. in Crabronidae, 194 spp. in Sphecidae), plus 190 species recorded from Nearctic Mexico (168 spp. in Crabronidae, 22 spp. in Sphecidae). The former Sphecidae (sensu Menke, 1997 and auct.) is divided in two families: Crabronidae (Astatinae, Bembicinae, Crabroninae, Pemphredoninae and Philanthinae) and Sphecidae (Ampulicinae and Sphecinae). The following subspecies are elevated to species: Podium aureosericeum Kohl, 1902; Podium bugabense Cameron, 1888. New names are proposed for the following junior homonyms: Cerceris modica new name for Cerceris modesta Smith, 1873, non Smith, 1856; Liris formosus new name for Liris bellus Rohwer, 1911, non Lepeletier, 1845; Liris inca new name for Liris peruanus Brèthes, 1926 non Brèthes, 1924; and Trypoxylon guassu new name for Trypoxylon majus Richards, 1934 non Trypoxylon figulus var. majus Kohl, 1883. KEYWORDS: Hymenoptera, Sphecidae, Crabronidae, Catalog, Taxonomy, Systematics, Nomenclature, New Name, Distribution. INTRODUCTION years ago and it is badly outdated now. Bohart and Menke (1976) cleared and updated most of the This catalog arose from the necessity to taxonomy of the spheciform wasps, complemented assess the present taxonomical knowledge of the by a series of errata sheets started by Menke and Neotropical spheciform wasps1, the Crabronidae Bohart (1979) and continued by Menke in the and Sphecidae. -

Magical Mekong: New Species Discoveries 2014
MAGICAL MEKONG: NEW SPECIES DISCOVERIES 2014 © Tom Gray / WWF-Greater Mekong Introduction An incredible 139 new species were discovered in the Greater Mekong region in 2014, including 90 plants, 23 reptiles, 16 amphibians, nine fish, and one mammal. The Greater Mekong Region (Cambodia, Laos, Myanmar, Thailand, and Vietnam) teems with life. Irrawaddy dolphins splash in the Mekong River, wild elephants and tigers roam Thailand’s forests, and giant ibises stalk the watering holes of Cambodia’s Eastern Plains Landscape. 1, 2, 3 In total, over 430 mammal species, 800 reptiles and amphibians, 1,200 birds, 1,100 fish and 20,000 plant species call this region home. 4 Every year, scientists describe new species increasing this tally and highlighting how much more is left to discover: between 1997 and 2014, 2,216 new species were discovered.5,6,7,8,9,10 In 2014, new species included a soul-sucking “dementor” wasp, a color-changing thorny frog, a stealthy wolf snake, the 10,000th reptile species discovered in the world, a bat with remarkable fangs, a new crocodile newt, a feathered coral, four Thai “Princess” moths, the world’s second-longest insect, and two orchids discovered through the wildlife trade. This incredible biodiversity underpins life for the Greater Mekong’s people. The Mekong’s fertile waters generate an estimated 2.6 million tonnes of fish per year – up to 25 percent of the global freshwater catch – and replenish the farms and rice paddies along its course with nutrient rich sediment. Forests and wetlands provide the raw materials for industry, purify the air and water, and protect towns and cities against natural disasters like floods and storms. -

UNIVERSITY of CALIFORNIA RIVERSIDE Venomics And
UNIVERSITY OF CALIFORNIA RIVERSIDE Venomics and Functional Analysis of Venom From the Emerald Jewel Wasp, Ampulex compressa A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Biochemistry and Molecular Biology by Ryan Scott Arvidson June 2016 Dissertation Committee: Dr. Michael E. Adams, Chairperson Dr. Jason Stajich Dr. Anandasankar Ray Copyright by Ryan Scott Arvidson 2016 The Dissertation of Ryan Scott Arvidson is approved: _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ Committee Chairperson University of California, Riverside Acknowledgements Given the adage that “Science does not happen in a vacuum”, I would like to first acknowledge those that contributed to the data presented in this dissertation and who are contributing authors in manuscripts in which this data is to be published. Victor Landa spent considerable time photographic and filming A. compressa in the lab which led to beautiful pictures, as is used in the introductory chapter, and an informative video that is available on the lab’s website (ampulex.ucr.edu). Victor also organized and obtained head capsule size data, and dissected mandibles from A. compressa larva and prepared them for electron microscopy. I would also like to thank Victor for is maintenance of the wasp colony. Sarah Frankenberg dissected and imaged cockroaches containing pupated wasps demonstrating that A. compressa larva is selective in which organs it consumes before pupating. Maayan Kaiser, who at the time of writing this dissertation was a Ph.D. student in the laboratory of Fredric Libersat, at the Ben- Gurion University, Beersheba, Israel, contributed her venom proteomics data in collaboration to generate the A. -

Hawaiian Entomological Society
PROCEEDINGS OF THE Hawaiian Entomological Society Editor Emeritus, O. H. Swezey Vol. XII, No. 1 For the Year 1943 August, 1944 This issue of the Proceedings is the first ever to appear without Mr. O. H. Swezey as its editor. After nearly forty years, at his own request, Mr. Swezey has relinquished the direction of this periodical in order to devote himself more fully to his studies of the native Hawaiian insect fauna. Under his editorship the Pro ceedings attained a position unique among journals of its kind, and constitutes an enduring monument to Mr. Swezey's long devotion to Hawaiian entomology. The amount of work he devoted to the production of the Proceedings cannot be fully appreciated by most of us. We know, however, that he has given to it the same fresh interest and zest which all phases of entomology claim from him. For the Society we wish to express our appreciation of his long and efficient service as editor, and the hope that as Editor Emeritus, he will continue for many years to counsel his successors on the editorial committee. JANUARY 11, 1943 The 445th meeting was held at the H.S.P.A. Experiment Sta tion on Monday, January 11, at 2:00 p.m., with President Hold- away in the chair. Members present: Messrs. Faxon, Fullaway, Holdaway, Krauss, Look, Marlowe, McBride, Nishida, Pemberton, Rosa, Sakimura, Stains, Swezey, Van Zwaluwenburg, Williams and Zimmerman. Visitor: Lt. R. L. Doutt, U.S.N. NOTES AND EXHIBITIONS Records from Kauai—Mr. Krauss presented the following data on material collected on Kauai: Stenotrupis marshalli Zimmerman; one specimen of this small weevil was taken at Lihue on October 18, 1942. -

Wasps and Bees in Southern Africa
SANBI Biodiversity Series 24 Wasps and bees in southern Africa by Sarah K. Gess and Friedrich W. Gess Department of Entomology, Albany Museum and Rhodes University, Grahamstown Pretoria 2014 SANBI Biodiversity Series The South African National Biodiversity Institute (SANBI) was established on 1 Sep- tember 2004 through the signing into force of the National Environmental Manage- ment: Biodiversity Act (NEMBA) No. 10 of 2004 by President Thabo Mbeki. The Act expands the mandate of the former National Botanical Institute to include respon- sibilities relating to the full diversity of South Africa’s fauna and flora, and builds on the internationally respected programmes in conservation, research, education and visitor services developed by the National Botanical Institute and its predecessors over the past century. The vision of SANBI: Biodiversity richness for all South Africans. SANBI’s mission is to champion the exploration, conservation, sustainable use, appreciation and enjoyment of South Africa’s exceptionally rich biodiversity for all people. SANBI Biodiversity Series publishes occasional reports on projects, technologies, workshops, symposia and other activities initiated by, or executed in partnership with SANBI. Technical editing: Alicia Grobler Design & layout: Sandra Turck Cover design: Sandra Turck How to cite this publication: GESS, S.K. & GESS, F.W. 2014. Wasps and bees in southern Africa. SANBI Biodi- versity Series 24. South African National Biodiversity Institute, Pretoria. ISBN: 978-1-919976-73-0 Manuscript submitted 2011 Copyright © 2014 by South African National Biodiversity Institute (SANBI) All rights reserved. No part of this book may be reproduced in any form without written per- mission of the copyright owners. The views and opinions expressed do not necessarily reflect those of SANBI. -

Hymenoptera: Sphecoidea)
The Great Lakes Entomologist Volume 21 Number 2 - Summer 1988 Number 2 - Summer Article 6 1988 June 1988 Records of Ampulicidae in Michigan (Hymenoptera: Sphecoidea) Mark F. O'Brien University of Michigan Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation O'Brien, Mark F. 1988. "Records of Ampulicidae in Michigan (Hymenoptera: Sphecoidea)," The Great Lakes Entomologist, vol 21 (2) Available at: https://scholar.valpo.edu/tgle/vol21/iss2/6 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. O'Brien: Records of Ampulicidae in Michigan (Hymenoptera: Sphecoidea) 1988 THE GREAT LAKES ENTOMOLOGIST 81 RECORDS OF AMPULICIDAE IN MICHIGAN (HYMENOPTERA: SPHECOIDEA) Mark F. O'Brienl ABSTRACT Two species of Ampulicidae, Dolichurus greenei and Ampule.>; canaliculata, are reported from Michigan for the first time. To date, there have been no published records of Ampulicidae from Michigan. The following records are new for the state and extend the known ranges considerably. Specimens are deposited in the University of Michigan Museum of Zoology (UMMZ), Michigan State University Collection (MSU), and the collection of D. P. Cowan (DPC). Dolichurus greenei Rohwer: MICHIGAN: Kalamazoo Co., T2S, RI2W, Sec. 7, 19 July-3 August, D. P. Cowan (all males), (UMMZ); St. Joseph Co., Klinger Lake, 30 July 1983, Malaise Trap, D. C. L. Gosling (26 males, 4 females) (UMMZ). -

Sphecos: a Forum for Aculeate Wasp Researchers
APRIL 1991 SPHECOS A FORUM FOR ACUlEATE WASP. RESEARCHERS MINUTIAE FROM THE ty• of digger wasps had a slightly une MUD D'AUB ARNOLDS. MENKE, Edhor ven distribution while the •nesting Tony Nuhn, Assistant Editor com Systematic Entomology Labratory munity• had a more patchy distnbution. Still no official word from the old Agricultural Research Senrice,USDA Sphecid communHies were more di· BMNH regarding personnel changes, c/o National Museum of Natural History verse on patches w~h relatively low but as of last November, Nigel Fergus Smithsonian I1Stitution, Washington, DC 20560 plant diversHy and cover. Diversity de· FAX: (202) son (a cynipoidist) was put in charge 786-9422 Phone: (202) 382-t803 creased in response to watering and of Coleoptera. Nigel informed me that watering combined wHh mechanical iso Tom Huddleston is now in charge of lation and increased after removal oi Hymenoptera. By the time you receive the upper layer of soil and plants. this issue of Sphecos, Mick Day may RESEARCH NEWS no longer be employed at The Natural lynn Kimsey (Dept. of Entomology, Alexander V. Antropov History Museum (aka BMNH). (Zoological Univ. of California. Davis, CA 95616, Museum of the Moscow lomonosov George Eickwort of Cornell Universi USA) reports "I am revising the wasp State ty is the President-elect of the Interna University, Herzen Street 6, Mos family Tiphiidae for the world, and have cow K-9 I tional Society of Hymenopterists. The 03009 USSR) has described begun sorting all of our miscellaneous a new genus of Crabroninae Society's second quadrennial meeting from Bra tiphiid wasps to genus and species. -

Identification and Ecology of Wasps (Apocrita: Hymenoptera) of Dhaka City
Bangladesh J. Zool. 48(1): 37-44, 2020 ISSN: 0304-9027 (print) 2408-8455 (online) IDENTIFICATION AND ECOLOGY OF WASPS (APOCRITA: HYMENOPTERA) OF DHAKA CITY Tangin Akter*, Jannat Ara Jharna, Shanjida Sultana, Soheli Akhter and Shefali Begum Department of Zoology, University of Dhaka, Dhaka-1000, Bangladesh Abstract: During the study period a total 351 wasp was collected from three different areas of Dhaka city viz Curzon Hall, Ramna Park and Sher-e-Bangla Agricultural University from October 2017 to May 2018. Among them 14 species belonging to four families-Ampulicidae, Sphecidae, Vespidae and Scoliidae were identified. The species were Ampulex compressa, Chalybion bengalense, Scoliasp., Laeviscolia frontalis, Delta esuriens, Rhynchium quinque cintum, Antodynerus flavescens, Parapolybiavaria sp., Ropalidia marginata, Polistes olivaceus, Polistes watti, Polistes stigma, Vespa tropica, and Vespa affinis. Standard taxonomic keys and sharp perception of outside morphology like head, wing venation, antennal sort, physical coloration etc. of the wasps were examined to identify them. Maximum of the distinguished species were beneath the family vespidae (72%). In the present study, it was observed that the maximum number of wasps were collected in May (29.63%). The richness of wasp species was more plenteousin Curzon Hall area (47.58%) than the Sher-e-Bangla Agricultural University area (40.17%) and was less abundant in Ramna park (12.25%). The main reason for finding more richness of wasp species in Curzon Hall area was the presence of various types of hedging plants than other two areas as the wasps were found to prefer hedging plants for foraging. It was also observed that Polistes olivaceus (21.93%)was the most abundant and Chalybion bengalense was (0.85%) the least abundant species in the study areas. -
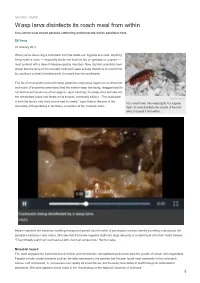
Wasp Larva Disinfects Its Roach Meal from Within Live-Action View Shows Parasite Slathering Antibacterials Inside Paralised Host
NATURE | NEWS Wasp larva disinfects its roach meal from within Live-action view shows parasite slathering antibacterials inside paralised host. Ed Yong 07 January 2013 When you're devouring a cockroach from the inside out, hygiene is a must. Anything living inside a roach — especially inside one that has fed on garbage or corpses — must contend with a slew of disease-causing microbes. Now, German scientists have shown that the larva of the emerald cockroach wasp actively disinfects its roach host by exuding a cocktail of antibacterial chemicals from its mouthparts. The life of an emerald cockroach wasp (Ampulex compressa) begins on an American cockroach (Periplaneta americana) that the mother wasp has stung, dragged back to her burrow and stuck one of her eggs to. Upon hatching, the wasp larva burrows into the immobilized roach and feasts on its innards, eventually killing it. “The cockroach Gudrun Herzner is both the larva’s only food source and its cradle,” says Gudrun Herzner of the For a roach lover, this wasp (right) is a hygiene University of Regensburg in Germany, a member of the research team. freak: its larva disinfects the innards of the host while it devours it from within. Herzner watched the larvae by installing transparent panels into the sides of parasitized roaches, literally providing a window on the parasite’s behaviour (see video). She saw that the larvae regularly slathered large amounts of a clear liquid onto their hosts' tissues. “They virtually soak their cockroaches with chemical compounds,” Herzner says. Microbial hazard The team analysed the liquid and found mellein and micromolide, two substances that can slow the growth of certain microorganisms. -

Insect Behavioral Change and the Potential Contributions of Neuroinflammation—A Call for Future Research
G C A T T A C G G C A T genes Review Insect Behavioral Change and the Potential Contributions of Neuroinflammation—A Call for Future Research Colleen A. Mangold 1,2 and David P. Hughes 1,2,3,* 1 Department of Entomology, College of Agricultural Sciences, Pennsylvania State University, University Park, State College, PA 16802, USA; [email protected] 2 Center for Infectious Disease Dynamics, Huck Institutes of the Life Sciences, Pennsylvania State University, University Park, State College, PA 16802, USA 3 Department of Biology, Eberly College of Science, Pennsylvania State University, University Park, State College, PA 16802, USA * Correspondence: [email protected] Abstract: Many organisms are able to elicit behavioral change in other organisms. Examples include different microbes (e.g., viruses and fungi), parasites (e.g., hairworms and trematodes), and parasitoid wasps. In most cases, the mechanisms underlying host behavioral change remain relatively unclear. There is a growing body of literature linking alterations in immune signaling with neuron health, communication, and function; however, there is a paucity of data detailing the effects of altered neuroimmune signaling on insect neuron function and how glial cells may contribute toward neuron dysregulation. It is important to consider the potential impacts of altered neuroimmune communica- tion on host behavior and reflect on its potential role as an important tool in the “neuro-engineer” toolkit. In this review, we examine what is known about the relationships between the insect immune and nervous systems. We highlight organisms that are able to influence insect behavior and discuss possible mechanisms of behavioral manipulation, including potentially dysregulated neuroimmune Citation: Mangold, C.A.; Hughes, communication. -

Life History of the Emerald Jewel Wasp Ampulex Compressa
JHR 63: 1–13 (2018) Life History of the Emerald Jewel Wasp Ampulex compressa 1 doi: 10.3897/jhr.63.21762 RESEARCH ARTICLE http://jhr.pensoft.net Life History of the Emerald Jewel Wasp Ampulex compressa Ryan Arvidson1,3, Victor Landa2, Sarah Frankenberg3, Michael E. Adams1,2,3 1 Graduate Program in Biochemistry and Molecular Biology, University of California, Riverside, CA, 92521, USA 2 Department of Cell Biology and Neuroscience, University of California, Riverside, CA 92521, USA 3 Department of Entomology, University of California, Riverside, CA 92521, USA Corresponding author: Michael E. Adams ([email protected]) Academic editor: M. Ohl | Received 20 October 2017 | Accepted 11 April 2018 | Published 30 April 2018 http://zoobank.org/0601867A-7BA7-4BE4-B2CA-9C50A6361082 Citation: Arvidson R, Landa V, Frankenberg S, Adams ME (2018) Life History of the Emerald Jewel Wasp Ampulex compressa. Journal of Hymenoptera Research 63: 1–13. https://doi.org/10.3897/jhr.63.21762 Abstract The Emerald Jewel Wasp Ampulex compressa (Fabricius) is an endoparasitoid of the American cockroach Periplaneta americana (Linnaeus). Its host subjugation strategy is unusual in that envenomation is directed into the host central nervous system, eliciting a long-term behavior modification termed hypokinesia, turning stung cockroaches into a lethargic and compliant, but not paralyzed, living food supply for wasp offspring.A. compressa manipulates hypokinesic cockroaches into a burrow, where it oviposits a single egg onto a mesothoracic leg, hatching three days later. Herein we describe the life history and developmental timing of A. compressa. Using head capsule measurements and observations of mandibular morphology, we found that the larvae develop through three instars, the first two ectoparasitoid, and the third exclusively endoparasitoid.