The Nucleocapsid of the Hepatitis B Virus: a Remarkable Immunogenic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Producing Vaccines Against Enveloped Viruses in Plants: Making the Impossible, Difficult
Review Producing Vaccines against Enveloped Viruses in Plants: Making the Impossible, Difficult Hadrien Peyret , John F. C. Steele † , Jae-Wan Jung, Eva C. Thuenemann , Yulia Meshcheriakova and George P. Lomonossoff * Department of Biochemistry and Metabolism, John Innes Centre, Norwich NR4 7UH, UK; [email protected] (H.P.); [email protected] (J.F.C.S.); [email protected] (J.-W.J.); [email protected] (E.C.T.); [email protected] (Y.M.) * Correspondence: [email protected] † Current address: Piramal Healthcare UK Ltd., Piramal Pharma Solutions, Northumberland NE61 3YA, UK. Abstract: The past 30 years have seen the growth of plant molecular farming as an approach to the production of recombinant proteins for pharmaceutical and biotechnological uses. Much of this effort has focused on producing vaccine candidates against viral diseases, including those caused by enveloped viruses. These represent a particular challenge given the difficulties associated with expressing and purifying membrane-bound proteins and achieving correct assembly. Despite this, there have been notable successes both from a biochemical and a clinical perspective, with a number of clinical trials showing great promise. This review will explore the history and current status of plant-produced vaccine candidates against enveloped viruses to date, with a particular focus on virus-like particles (VLPs), which mimic authentic virus structures but do not contain infectious genetic material. Citation: Peyret, H.; Steele, J.F.C.; Jung, J.-W.; Thuenemann, E.C.; Keywords: alphavirus; Bunyavirales; coronavirus; Flaviviridae; hepatitis B virus; human immunode- Meshcheriakova, Y.; Lomonossoff, ficiency virus; Influenza virus; newcastle disease virus; plant molecular farming; plant-produced G.P. -

Characterization of Antibodies to Human Immunodeficiency Viral Proteins in the Sera of HIV Infected and Non HIV Infected Hbsag Seropositive Patients
Global Journal of Health Science Vol. 2, No. 1; April 2010 Characterization of Antibodies to Human Immunodeficiency Viral Proteins in the Sera of HIV Infected and Non HIV Infected HBsAg Seropositive Patients Dr. Mathew Folaranmi OLANIYAN (Ph. D) School of Medical Laboratory Technology, Baptist Medical Centre P.M. B. 43, Saki – Oyo State, Nigeria Tel: 234-805-224-8019 E-mail: [email protected] Abstract This work was designed to characterize the HIV viral antibodies in HIV infected and non-HIV infected HBsAg seropositive patients. Fifty non HIV infected (male = 25; female = 25)and 50 HIV infected HBsAg seropositive patients (male – n = 25; female – n = 25) aged 6 – 64 years recruited from the medical outpatient department of Baptist Medical Centre, Saki – Oyo State – Nigeria were investigated as test subjects. Fifty apparently healthy HIV and HBsAg seronegative individuals (male = 25; female = 25) aged 4 – 72years were recruited as control subjects. All subjects were counseled and were subjected to HBsAg and HIV immunoassays by Enzyme Linked Immunosorbent Assay and Western blot assay. All subjects were monitored for twelve months. The subjects were investigated on recruitment and 12months after recruitment. The result obtained indicated higher frequency of occurrence of each of the HIV antibodies to each of the viral proteins in HIV infected HBsAg seropositive patients than in non HIV infected HBsAg seropositive patients (94% vs 0% (gp 160,), 84% vs 0% (gp 120), 94% vs 4% (p66), 94% vs 4% (p51), 84% vs 0% (gp 41), 94% vs 0% (p31), 100% vs 24% (p24) and 84% vs 6% (p17) during the first bleeding. -

Enveloped Viruses Distinct from HBV Induce Dissemination of Hepatitis D Virus in Vivo
ARTICLE https://doi.org/10.1038/s41467-019-10117-z OPEN Enveloped viruses distinct from HBV induce dissemination of hepatitis D virus in vivo Jimena Perez-Vargas 1, Fouzia Amirache1, Bertrand Boson1, Chloé Mialon1, Natalia Freitas1, Camille Sureau2, Floriane Fusil1 & François-Loïc Cosset 1 Hepatitis D virus (HDV) doesn’t encode envelope proteins for packaging of its ribonucleo- protein (RNP) and typically relies on the surface glycoproteins (GPs) from hepatitis B virus 1234567890():,; (HBV) for virion assembly, envelopment and cellular transmission. HDV RNA genome can efficiently replicate in different tissues and species, raising the possibility that it evolved, and/ or is still able to transmit, independently of HBV. Here we show that alternative, HBV- unrelated viruses can act as helper viruses for HDV. In vitro, envelope GPs from several virus genera, including vesiculovirus, flavivirus and hepacivirus, can package HDV RNPs, allowing efficient egress of HDV particles in the extracellular milieu of co-infected cells and sub- sequent entry into cells expressing the relevant receptors. Furthermore, HCV can propagate HDV infection in the liver of co-infected humanized mice for several months. Further work is necessary to evaluate whether HDV is currently transmitted by HBV-unrelated viruses in humans. 1 CIRI—Centre International de Recherche en Infectiologie, Univ Lyon, Université Claude Bernard Lyon 1, Inserm, U1111, CNRS, UMR5308, ENS Lyon, 46 allée d’Italie, F-69007 Lyon, France. 2 Molecular Virology laboratory, Institut National de la Transfusion Sanguine (INTS), CNRS Inserm U1134, 6 rue Alexandre Cabanel, F-75739 Paris, France. Correspondence and requests for materials should be addressed to F.-L.C. -

Developing a Novel Hepatitis B Core – Based Antigen Presentation System
Developing a novel hepatitis B core – based antigen presentation system by Hadrien Peyret This thesis is submitted in partial fulfilment of the requirements of the degree of Doctor of Philosophy at the University of East Anglia. John Innes Centre, Norwich, UK September 2014 © This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with the author and that use of any information derived therefrom must be in accordance with current UK Copyright Law. In addition, any quotation of extract must include full attribution. 1 Declaration Declaration I hereby certify that the work contained within this thesis is my own original work, except where due reference is made to other contributing authors. This thesis is submitted for the degree of Doctor of Philosophy at the University of East Anglia and has not been submitted to this or any other university for any other qualification. Hadrien Peyret ii Abstract Abstract Plant-produced proteins of pharmaceutical interest are beginning to reach the market, and the advantages of transient plant expression systems are gaining increasing recognition. In parallel, the use of virus-like particles (VLPs) has become standard in vaccine design. The pEAQ-HT vector derived from the cowpea mosaic virus – based CPMV-HT expression system has been shown to allow the production of large amounts of recombinant proteins, including VLPs, in Nicotiana benthamiana. Moreover, previous work demonstrated that a tandem fusion of the core antigen (HBcAg) of hepatitis B virus (HBV) could direct the formation of core-like particles (CLPs) in plants. -
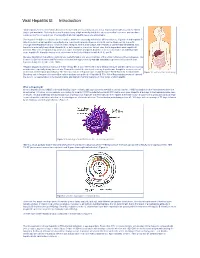
Viral Hepatitis B: Introduction
Viral Hepatitis B: Introduction “Viral hepatitis," refers to infections that affect the liver and are caused by viruses. It is a major public health issue in the United States and worldwide. Not only does viral hepatitis carry a high morbidity, but it also stresses medical resources and can have severe economic consequences. The majority of all viral hepatitis cases are preventable. Viral hepatitis includes five distinct disease entities, which are caused by at least five different viruses. Hepatitis A and hepatitis B (infectious and serum hepatitis, respectively) are considered separate diseases and both can be diagnosed by a specific serologic test. Hepatitis C and E comprise a third category, each a distinct type, with Hepatitis C parenterally transmitted, and hepatitis E enterically transmitted. Hepatitis D, or delta hepatitis, is another distinct virus that is dependent upon hepatitis B infection. This form of hepatitis may occur as a super-infectionin a hepatitis B carrier or as a co-infection in an individual with acute hepatitis B. Hepatitis viruses most often found in the United States include A, B, C, and D. Because fatality from hepatitis is relatively low, mortality figures are a poor indicator of the actual incidence of these diseases. The Centers for Disease Control and Prevention estimated that approximately 400,000–600,000 people were infected with viral hepatitis during the decade of the 1990s. Hepatitis plagued mankind as early as the fifth century BC. It was referenced in early biblical literature and described as occurring in outbreaks, especially during times of war. Toward the end of the nineteenth century, hepatitis was thought to occur as a result of infection of the hepatic parenchyma. -

Current Opinion in Infectious Diseases Was Launched in 1988
June 2007, Volume 20, Issue 3,pp.237-344 Editorial introductions vii Editorial introductions. Paediatric and neonatal infections 237 Adverse events following immunization: perception and evidence. Jan Bonhoeffer; Ulrich Heininger 247 Managing congenital syphilis again? The more things change [horizontal ellipsis]. Rana Chakraborty; Suzanne Luck 253 Rotavirus vaccines in developed countries. Jim P Buttery; Carl Kirkwood 259 The burden of influenza in children. Mary Iskander; Robert Booy; Stephen Lambert 264 T cell-based diagnosis of childhood tuberculosis infection. Ajit Lalvani; Kerry A Millington 272 Aseptic meningitis. Bonita E Lee; H Dele Davies Pathogenesis and immune response 278 Herpesviral-bacterial synergy in the pathogenesis of human periodontitis. Jørgen Slots 284 Leptospirosis: pathogenesis, immunity, and diagnosis. Raghavan UM Palaniappan; Subbupoongothai Ramanujam; Yung-Fu Chang 293 Experimental infections with West Nile virus. Richard A Bowen; Nicole M Nemeth 298 The role of superantigens of group A Streptococcus and Staphylococcus aureus in Kawasaki disease. Kousaku Matsubara; Takashi Fukaya 304 Distinction between bacterial and viral infections. Jari Nuutila; Esa-Matti Lilius 311 New concepts in vaccine development in malaria. Bernard N Kanoi; Thomas G Egwang Current World Literature Bibliography 317 Current World Literature. Editorial introductions Current Opinion in Infectious Diseases was launched in 1988. Utah, Dr Stevens com- It is part of a successful series of review journals whose pleted an Infectious Dis- unique format is designed to provide a systematic and ease Fellowship at Brooke critical assessment of the literature as presented in the Army Medical Center in many primary journals. The field of infectious diseases is San Antonio, Texas, and divided into 12 sections that are reviewed once a year. -

Will Plant-Based Vaccines Be the Answer?
Review Combating Human Viral Diseases: Will Plant-Based Vaccines Be the Answer? Srividhya Venkataraman 1,*, Kathleen Hefferon 1, Abdullah Makhzoum 2 and Mounir Abouhaidar 1 1 Virology Laboratory, Department of Cell & Systems Biology, University of Toronto, Toronto, ON M5S 3B2, Canada; [email protected] (K.H.); [email protected] (M.A.) 2 Department of Biological Sciences & Biotechnology, Botswana International University of Science & Technology, Palapye, Botswana; [email protected] * Correspondence: [email protected] Abstract: Molecular pharming or the technology of application of plants and plant cell culture to manufacture high-value recombinant proteins has progressed a long way over the last three decades. Whether generated in transgenic plants by stable expression or in plant virus-based transient ex- pression systems, biopharmaceuticals have been produced to combat several human viral diseases that have impacted the world in pandemic proportions. Plants have been variously employed in expressing a host of viral antigens as well as monoclonal antibodies. Many of these biopharmaceuti- cals have shown great promise in animal models and several of them have performed successfully in clinical trials. The current review elaborates the strategies and successes achieved in generating plant-derived vaccines to target several virus-induced health concerns including highly commu- nicable infectious viral diseases. Importantly, plant-made biopharmaceuticals against hepatitis B virus (HBV), hepatitis C virus (HCV), the cancer-causing virus human papillomavirus (HPV), human Citation: Venkataraman, S.; immunodeficiency virus (HIV), influenza virus, zika virus, and the emerging respiratory virus, Hefferon, K.; Makhzoum, A.; Abouhaidar, M. Combating Human severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) have been discussed. -

Role of Humoral Immunity Against Hepatitis B Virus Core Antigen in the Pathogenesis of Acute Liver Failure
Role of humoral immunity against hepatitis B virus core antigen in the pathogenesis of acute liver failure Zhaochun Chena, Giacomo Diazb, Teresa Pollicinoa,c, Huaying Zhaod, Ronald E. Englea, Peter Schuckd, Chen-Hsiang Shene, Fausto Zambonif, Zhifeng Longg, Juraj Kabath, Davide De Battistaa, Kevin W. Bocki, Ian N. Moorei, Kurt Wollenbergj, Cinque Sotoe, Sugantha Govindarajank, Peter D. Kwonge, David E. Kleinerl, Robert H. Purcella,1, and Patrizia Farcia,1 aHepatic Pathogenesis Section, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892; bDepartment of Biomedical Sciences, University of Cagliari, 09124 Cagliari, Italy; cDepartment of Human Pathology, University of Messina, 98122 Messina, Italy; dLaboratory of Cellular Imaging and Macromolecular Biophysics, National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, Bethesda, MD 20892; eVaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892; fLiver Transplantation Center, Azienda Ospedaliera Brotzu, 09134 Cagliari, Italy; gPersonal Diagnostix, Inc., Gaithersburg, MD 20879; hBiological Imaging Facility/Research Technologies Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892; iComparative Medicine Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892; jBioinformatics and Computational -

Setting New Standards for Vaccine Development & Testing
Setting New Standards For Vaccine Development & Testing "The impact of vaccination on the health of the world's peoples is hard to exaggerate. With the exception of safe water, no other modality, not even antibiotics, has had such a major effect on mortality reduction and population growth." Since the early recognition of vaccines by Edward Jenner in 1796, millions of lives have been saved by a variety of bacterial and human vaccines. Until recently, most vaccines were aimed at infants and children, but adolescents and adults (e.g., obesity and drug abuse) are increasingly being targeted. New methods of administering vaccines such as skin patches, aerosols via inhalation devices, and eating genetically engineered plants. Attempts are being made to develop vaccines to help cure chronic infections, as opposed to preventing disease. Vaccines are being developed to defend against bioterrorist attacks such as anthrax, plague, and smallpox. Malarial alone kills millions every year. There is an urgent need to develop effective vaccines for Malaria and HIV etc. Biotech and vaccine manufactures have paid more attention to produce vaccine but fewer companies have developed reliable tests to determine efficacy of vaccines in animals and humans. ADI is at forefront of developing newer tests for a variety of old and new vaccines. It is for the first time that one can identify and measure the vaccine after adsorbing it on Alum. This brochure presents a brief summary of antibodies, reagents and test methods available for various vaccines. Page # Vaccines: Custom Testing 2 Malaria Vaccines 3-4 HPV Vaccines 5-6 Anthrax Vaccines 7-8 Breast Cancer Vaccines 9-10 Diphtheria Vaccines 11 Tetanus Vaccines 12 Pertussis Vaccines 13-14 VacciGel ELISAs 15-16 Rabies Vaccines 17 Polio Vaccines 18 Measles Vaccines 19 Mumps Vaccines 20 Rubella Vaccines 21 H. -

XXVI Brazilian Congress of Virology
Virus Reviews and Research Journal of the Brazilian Society for Virology Volume 20, October 2015, Supplement 1 Annals of the XXVI Brazilian Congress of Virology & X Mercosur Meeting of Virology October, 11 - 14, 2015, Costão do Santinho Resort, Florianópolis, Santa Catarina, Brazil Editors Edson Elias da Silva Fernando Rosado Spilki BRAZILIAN SOCIETY FOR VIROLOGY BOARD OF DIRECTORS (2015-2016) Officers Area Representatives President: Dr. Bergmann Morais Ribeiro Basic Virology (BV) Vice-President: Dr. Célia Regina Monte Barardi Dr. Luciana Jesus da Costa, UFRJ (2015 – 2016) First Secretary: Dr. Fernando Rosado Spilki Dr. Luis Lamberti Pinto da Silva, USP-RP (2015 – 2016) Second Secretary: Dr. Mauricio Lacerda Nogueira First Treasurer: Dr. Alice Kazuko Inoue Nagata Environmental Virology (EV) Second Treasurer: Dr. Zélia Inês Portela Lobato Dr. Adriana de Abreu Correa, UFF (2015 – 2016) Executive Secretary: Dr Fabrício Souza Campos Dr. Jônatas Santos Abrahão, UFMG (2015 – 2016 Human Virology (HV) Fiscal Councilors Dr. Eurico de Arruda Neto, USP-RP (2015 – 2016) Dr. Viviane Fongaro Botosso Dr. Paula Rahal, UNESP (2015 – 2016) Dr. Davis Fernandes Ferreira Dr. Maria Ângela Orsi Immunobiologicals in Virology (IV) Dr. Flávio Guimarães da Fonseca, UFMG (2015 – 2016) Dr. Jenner Karlisson Pimenta dos Reis, UFMG (2015 – 2016) Plant and Invertebrate Virology (PIV) Dr. Maite Vaslin De Freitas Silva, UFRJ (2015 – 2016) Dr. Tatsuya Nagata, UNB (2015 – 2016) Veterinary Virology (VV) Dr. João Pessoa Araújo Junior, UNESP (2015 – 2016) Dr. Marcos Bryan Heinemann, USP (2015 – 2016) Address Universidade Feevale, Instituto de Ciências da Saúde Estrada RS-239, 2755 - Prédio Vermelho, sala 205 - Laboratório de Microbiologia Molecular Bairro Vila Nova - 93352-000 - Novo Hamburgo, RS - Brasil Phone: (51) 3586-8800 E-mail: F.R.Spilki - [email protected] http://www.vrrjournal.org.br Organizing Committee Dr. -

Immunogenicity of Peptide Fusions to Hepatitis B Virus Core Antigen (Recombinant DNA/Serology/Animal Virus/Human Immunodeficiency Virus/Vaccdne) STEPHEN J
Proc. Nati. Acad. Sci. USA Vol. 86, pp. 6283-6287, August 1989 Immunology Immunogenicity of peptide fusions to hepatitis B virus core antigen (recombinant DNA/serology/animal virus/human immunodeficiency virus/vaccdne) STEPHEN J. STAHL*t AND KENNETH MURRAYt *Biogen S.A., CH-1211, Geneva 24, Switzerland; and tDepartment of Molecular Biology, University of Edinburgh, King's Buildings, Edinburgh EH9 3JR, Scotland Communicated by Frederick Sanger, May IS, 1989 ABSTRACT Several gene fusions have been constructed in is a derivative of E. coli K-12 W3110 (8), which harbors an which coding sequences for antigenic regions of the pre-S F'lac plasmid having a laclQ gene for overproduction of the sequences of hepatitis B virus, hepatitis B surface antigen, and lac repressor. Plasmid pRi-li, which directs the synthesis of the envelope protein of human immunodeficiency virus were HBcAg, has been described (6). Plasmid pBR1-Sa, which linked to the 3' end ofthat for the first 144 residues of hepatitis consists of the Hpa II 570-base-pair (bp) restriction fragment B core antigen. The sequences were expressed efficiently in containing the trp promoter-operator-attenuator region (9) Escherichia coli to give stable products that assembled to form cloned between the EcoRI and Sal I restriction sites of particles morphologically similar to hepatitis B core antigen pBR322, was the gift of K. Bertrand (Stanford University). itself. The products exhibited the antigenic and immunogenic The plasmid pHBV130 contains coding regions ofHBsAg and characteristics ofboth the hepatitis B core antigen epitopes and also has been described (10). the epitopes carried by the additional sequences, thus iMlustrat- Cloning Reagents and Techniques. -

HALVORSEN, B. and Qlrstavik,I.Lll Institute for Experimental
HALVORSEN, B. and QlRSTAVIK,I.lll THE INTERFERON SYSTEM: A NEW ASSAY AND ITS CLINICAL 11 Institute for Experimental Medical Research, Pediatric Dept. 14 IMPLICATIONS. STANLEY LEVIN AND THALIA HAHN*, Pediat and Mlcroblologlcal Dept., Ulleval Hospital, Oslo 1, Norway. ric Research Dept, Kaplan Hospital, Rehovot, Israel. Prevention of viral replication is a major function of the THE EFFECT OF HUMAN MILK FRACTION ON ROTAVIRUS AND Interferon (IF) system in man, and can be divided into 2 phases. E. COLI HEAT LABILE ENTEROTOXIN. Firstly, the production of IF by cells following stimulation by Breast fed Infants suffer less from gastrointestinal infections compared any of a number of inducers, including viruses; and secondly, the to artificially fed Infants. We have studied the protective effect of frac action of this IF on other cells, leading to the production of tions of milk from Ethiopian and Norwegian women against rotavlrus and anti-viral proteins which become activated when a virus enters the E. col! heat labile enterotoxin In vitro. Human milk was fractionated by cell, thereby preventing replication of the virus. We have devel ammonium sulphate precipitation (50%) and column chromatography oped an assay system which simultaneously examines the blood IF, (Ultrogel AcA 44). Rotavlrus specific antibodies were detected by the ability of mononuclear cells to produce both type I and type immunofluorescence in 15 out of 24 samples before fractionation and In :I IF, and whether the cells have been primed into an anti-viral 11 out of 13 of the concentrated immunoglobulin-rich fractions. Rotavirus state, Further this assav will show whether in the absence of in infection of LLC-MK2 cells was Inhibited by the concentrated Immuno vivo cell protection viral replication, extrinsic lF is able globulin-enriched milk fractions, as well as by some Immunoglobulin to prime the anti-viral state and at what dosage level.