Human DNA Virus Exploitation of the MAPK-ERK Cascade
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vaccinia Belongs to a Family of Viruses That Is Closely Related to the Smallpox Virus
VACCINIA INFECTION What is it? Vaccinia belongs to a family of viruses that is closely related to the smallpox virus. Because of the similarities between the smallpox and vaccinia viruses, the vaccinia virus is used in the smallpox vaccine. When this virus is used as a vaccine, it allows our immune systems to develop immunity against smallpox. The smallpox vaccine does not actually contain smallpox virus and cannot cause smallpox. Vaccination usually prevents smallpox infection for at least ten years. The vaccinia vaccine against smallpox was used to successfully eradicate smallpox from the human population. More recently, this virus has also become of interest due to concerns about smallpox being used as an agent of bioterrorism. How is the virus spread? Vaccinia can be spread by touching the vaccination site before it has fully healed or by touching clothing or bandages that have been contaminated with the live virus during vaccination. In this manner, vaccinia can spread to other parts of the body and to other individuals. It cannot be spread through the air. What are the symptoms of vaccinia? Vaccinia virus symptoms are similar to smallpox, but milder. Vaccinia may cause rash, fever, headache and body aches. In certain individuals, such as those with weak immune systems, the symptoms can be more severe. What are the potential side effects of the vaccinia vaccine for smallpox? Normal reactions are mild and go away without any treatment.These include: Soreness and redness in the arm where the vaccine was given Slightly swollen, sore glands in the armpits Low grade fever One in approximately three people will feel badly enough to miss school, work or recreational activities Trouble sleeping Serious reactions are not very common but can occur in about 1,000 in every 1 million people who are vaccinated for the first time. -

Cidofovir Activity Against Poxvirus Infections
Viruses 2010 , 2, 2803-2830; doi:10.3390/v2122803 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Cidofovir Activity against Poxvirus Infections Graciela Andrei * and Robert Snoeck Laboratory of Virology and Chemotherapy, Rega Institute for Medical Research, KULeuven, Minderboredersstraat 10, B-3000 Leuven, Belgium; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +32-16-337372; Fax: +32-16-337340. Received: 10 November 2010; in revised form: 9 December 2010 / Accepted: 10 December 2010 / Published: 22 December 2010 Abstract: Cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, HPMPC] is an acyclic nucleoside analog approved since 1996 for clinical use in the treatment of cytomegalovirus (CMV) retinitis in AIDS patients. Cidofovir (CDV) has broad-spectrum activity against DNA viruses, including herpes-, adeno-, polyoma-, papilloma- and poxviruses. Among poxviruses, cidofovir has shown in vitro activity against orthopox [vaccinia, variola (smallpox), cowpox, monkeypox, camelpox, ectromelia], molluscipox [molluscum contagiosum] and parapox [orf] viruses. The anti-poxvirus activity of cidofovir in vivo has been shown in different models of infection when the compound was administered either intraperitoneal, intranasal (aerosolized) or topically. In humans, cidofovir has been successfully used for the treatment of recalcitrant molluscum contagiosum virus and orf virus in immunocompromised patients. CDV remains a reference compound against poxviruses and holds potential for the therapy and short-term prophylaxis of not only orthopox- but also parapox- and molluscipoxvirus infections. Keywords: cidofovir; poxviruses; acyclic nucleoside analog 1. Introduction The antiviral activity of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC, cidofovir, CDV) (Figure 1) against human cytomegalovirus (HCMV) and other DNA viruses was first Viruses 2010 , 2 2804 reported in 1986 [1]. -

(12) Patent Application Publication (10) Pub. No.: US 2012/0009150 A1 WEBER Et Al
US 2012O009 150A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0009150 A1 WEBER et al. (43) Pub. Date: Jan. 12, 2012 (54) DIARYLUREAS FORTREATINGVIRUS Publication Classification INFECTIONS (51) Int. Cl. (76) Inventors: Olaf WEBER, Wulfrath (DE); st 2. CR Bernd Riedl, Wuppertal (DE) ( .01) A63/675 (2006.01) (21) Appl. No.: 13/236,865 A6II 3/522 (2006.01) A6IP 29/00 (2006.01) (22) Filed: Sep. 20, 2011 A6II 3/662 (2006.01) A638/14 (2006.01) Related U.S. Application Data A63L/7056 (2006.01) A6IP3L/2 (2006.01) (63) Continuation of application No. 12/097.350. filed on A6II 3/44 (2006.01) Nov. 3, 2008, filed as application No. PCTAEPO6/ A6II 3/52 (2006.01) 11693 on Dec. 6, 2006. O O (52) U.S. Cl. .......... 424/85.6; 514/350; 514/171; 514/81; (30) Foreign Application Priority Data 514/263.38: 514/263.4: 514/120: 514/4.3: Dec. 15, 2005 (EP) .................................. 05O274513 424/85.7; 514/43 Dec. 15, 2005 (EP). ... O5O27452.1 Dec. 15, 2005 (EP). ... O5O27456.2 Dec. 15, 2005 (EP). ... O5O27458.8 The present invention relates to pharmaceutical compositions Dec. 15, 2005 (EP) O5O27.460.4 for treating virus infections and/or diseases caused by virus Dec. 15, 2005 (EP) O5O27462.O infections comprising at least a diary1 urea compound option Dec. 15, 2005 (EP). ... O5O27465.3 ally combined with at least one additional therapeutic agent. Dec. 15, 2005 (EP). ... O5O274.67.9 Useful combinations include e.g. BAY 43-9006 as a diaryl Dec. -

Producing Vaccines Against Enveloped Viruses in Plants: Making the Impossible, Difficult
Review Producing Vaccines against Enveloped Viruses in Plants: Making the Impossible, Difficult Hadrien Peyret , John F. C. Steele † , Jae-Wan Jung, Eva C. Thuenemann , Yulia Meshcheriakova and George P. Lomonossoff * Department of Biochemistry and Metabolism, John Innes Centre, Norwich NR4 7UH, UK; [email protected] (H.P.); [email protected] (J.F.C.S.); [email protected] (J.-W.J.); [email protected] (E.C.T.); [email protected] (Y.M.) * Correspondence: [email protected] † Current address: Piramal Healthcare UK Ltd., Piramal Pharma Solutions, Northumberland NE61 3YA, UK. Abstract: The past 30 years have seen the growth of plant molecular farming as an approach to the production of recombinant proteins for pharmaceutical and biotechnological uses. Much of this effort has focused on producing vaccine candidates against viral diseases, including those caused by enveloped viruses. These represent a particular challenge given the difficulties associated with expressing and purifying membrane-bound proteins and achieving correct assembly. Despite this, there have been notable successes both from a biochemical and a clinical perspective, with a number of clinical trials showing great promise. This review will explore the history and current status of plant-produced vaccine candidates against enveloped viruses to date, with a particular focus on virus-like particles (VLPs), which mimic authentic virus structures but do not contain infectious genetic material. Citation: Peyret, H.; Steele, J.F.C.; Jung, J.-W.; Thuenemann, E.C.; Keywords: alphavirus; Bunyavirales; coronavirus; Flaviviridae; hepatitis B virus; human immunode- Meshcheriakova, Y.; Lomonossoff, ficiency virus; Influenza virus; newcastle disease virus; plant molecular farming; plant-produced G.P. -

Characterization of Antibodies to Human Immunodeficiency Viral Proteins in the Sera of HIV Infected and Non HIV Infected Hbsag Seropositive Patients
Global Journal of Health Science Vol. 2, No. 1; April 2010 Characterization of Antibodies to Human Immunodeficiency Viral Proteins in the Sera of HIV Infected and Non HIV Infected HBsAg Seropositive Patients Dr. Mathew Folaranmi OLANIYAN (Ph. D) School of Medical Laboratory Technology, Baptist Medical Centre P.M. B. 43, Saki – Oyo State, Nigeria Tel: 234-805-224-8019 E-mail: [email protected] Abstract This work was designed to characterize the HIV viral antibodies in HIV infected and non-HIV infected HBsAg seropositive patients. Fifty non HIV infected (male = 25; female = 25)and 50 HIV infected HBsAg seropositive patients (male – n = 25; female – n = 25) aged 6 – 64 years recruited from the medical outpatient department of Baptist Medical Centre, Saki – Oyo State – Nigeria were investigated as test subjects. Fifty apparently healthy HIV and HBsAg seronegative individuals (male = 25; female = 25) aged 4 – 72years were recruited as control subjects. All subjects were counseled and were subjected to HBsAg and HIV immunoassays by Enzyme Linked Immunosorbent Assay and Western blot assay. All subjects were monitored for twelve months. The subjects were investigated on recruitment and 12months after recruitment. The result obtained indicated higher frequency of occurrence of each of the HIV antibodies to each of the viral proteins in HIV infected HBsAg seropositive patients than in non HIV infected HBsAg seropositive patients (94% vs 0% (gp 160,), 84% vs 0% (gp 120), 94% vs 4% (p66), 94% vs 4% (p51), 84% vs 0% (gp 41), 94% vs 0% (p31), 100% vs 24% (p24) and 84% vs 6% (p17) during the first bleeding. -

Enveloped Viruses Distinct from HBV Induce Dissemination of Hepatitis D Virus in Vivo
ARTICLE https://doi.org/10.1038/s41467-019-10117-z OPEN Enveloped viruses distinct from HBV induce dissemination of hepatitis D virus in vivo Jimena Perez-Vargas 1, Fouzia Amirache1, Bertrand Boson1, Chloé Mialon1, Natalia Freitas1, Camille Sureau2, Floriane Fusil1 & François-Loïc Cosset 1 Hepatitis D virus (HDV) doesn’t encode envelope proteins for packaging of its ribonucleo- protein (RNP) and typically relies on the surface glycoproteins (GPs) from hepatitis B virus 1234567890():,; (HBV) for virion assembly, envelopment and cellular transmission. HDV RNA genome can efficiently replicate in different tissues and species, raising the possibility that it evolved, and/ or is still able to transmit, independently of HBV. Here we show that alternative, HBV- unrelated viruses can act as helper viruses for HDV. In vitro, envelope GPs from several virus genera, including vesiculovirus, flavivirus and hepacivirus, can package HDV RNPs, allowing efficient egress of HDV particles in the extracellular milieu of co-infected cells and sub- sequent entry into cells expressing the relevant receptors. Furthermore, HCV can propagate HDV infection in the liver of co-infected humanized mice for several months. Further work is necessary to evaluate whether HDV is currently transmitted by HBV-unrelated viruses in humans. 1 CIRI—Centre International de Recherche en Infectiologie, Univ Lyon, Université Claude Bernard Lyon 1, Inserm, U1111, CNRS, UMR5308, ENS Lyon, 46 allée d’Italie, F-69007 Lyon, France. 2 Molecular Virology laboratory, Institut National de la Transfusion Sanguine (INTS), CNRS Inserm U1134, 6 rue Alexandre Cabanel, F-75739 Paris, France. Correspondence and requests for materials should be addressed to F.-L.C. -

Developing a Novel Hepatitis B Core – Based Antigen Presentation System
Developing a novel hepatitis B core – based antigen presentation system by Hadrien Peyret This thesis is submitted in partial fulfilment of the requirements of the degree of Doctor of Philosophy at the University of East Anglia. John Innes Centre, Norwich, UK September 2014 © This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with the author and that use of any information derived therefrom must be in accordance with current UK Copyright Law. In addition, any quotation of extract must include full attribution. 1 Declaration Declaration I hereby certify that the work contained within this thesis is my own original work, except where due reference is made to other contributing authors. This thesis is submitted for the degree of Doctor of Philosophy at the University of East Anglia and has not been submitted to this or any other university for any other qualification. Hadrien Peyret ii Abstract Abstract Plant-produced proteins of pharmaceutical interest are beginning to reach the market, and the advantages of transient plant expression systems are gaining increasing recognition. In parallel, the use of virus-like particles (VLPs) has become standard in vaccine design. The pEAQ-HT vector derived from the cowpea mosaic virus – based CPMV-HT expression system has been shown to allow the production of large amounts of recombinant proteins, including VLPs, in Nicotiana benthamiana. Moreover, previous work demonstrated that a tandem fusion of the core antigen (HBcAg) of hepatitis B virus (HBV) could direct the formation of core-like particles (CLPs) in plants. -

Here, There, and Everywhere: the Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses
viruses Review Here, There, and Everywhere: The Wide Host Range and Geographic Distribution of Zoonotic Orthopoxviruses Natalia Ingrid Oliveira Silva, Jaqueline Silva de Oliveira, Erna Geessien Kroon , Giliane de Souza Trindade and Betânia Paiva Drumond * Laboratório de Vírus, Departamento de Microbiologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais: Belo Horizonte, Minas Gerais 31270-901, Brazil; [email protected] (N.I.O.S.); [email protected] (J.S.d.O.); [email protected] (E.G.K.); [email protected] (G.d.S.T.) * Correspondence: [email protected] Abstract: The global emergence of zoonotic viruses, including poxviruses, poses one of the greatest threats to human and animal health. Forty years after the eradication of smallpox, emerging zoonotic orthopoxviruses, such as monkeypox, cowpox, and vaccinia viruses continue to infect humans as well as wild and domestic animals. Currently, the geographical distribution of poxviruses in a broad range of hosts worldwide raises concerns regarding the possibility of outbreaks or viral dissemination to new geographical regions. Here, we review the global host ranges and current epidemiological understanding of zoonotic orthopoxviruses while focusing on orthopoxviruses with epidemic potential, including monkeypox, cowpox, and vaccinia viruses. Keywords: Orthopoxvirus; Poxviridae; zoonosis; Monkeypox virus; Cowpox virus; Vaccinia virus; host range; wild and domestic animals; emergent viruses; outbreak Citation: Silva, N.I.O.; de Oliveira, J.S.; Kroon, E.G.; Trindade, G.d.S.; Drumond, B.P. Here, There, and Everywhere: The Wide Host Range 1. Poxvirus and Emerging Diseases and Geographic Distribution of Zoonotic diseases, defined as diseases or infections that are naturally transmissible Zoonotic Orthopoxviruses. Viruses from vertebrate animals to humans, represent a significant threat to global health [1]. -
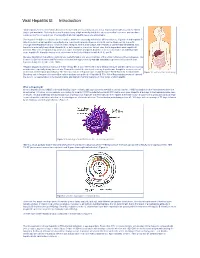
Viral Hepatitis B: Introduction
Viral Hepatitis B: Introduction “Viral hepatitis," refers to infections that affect the liver and are caused by viruses. It is a major public health issue in the United States and worldwide. Not only does viral hepatitis carry a high morbidity, but it also stresses medical resources and can have severe economic consequences. The majority of all viral hepatitis cases are preventable. Viral hepatitis includes five distinct disease entities, which are caused by at least five different viruses. Hepatitis A and hepatitis B (infectious and serum hepatitis, respectively) are considered separate diseases and both can be diagnosed by a specific serologic test. Hepatitis C and E comprise a third category, each a distinct type, with Hepatitis C parenterally transmitted, and hepatitis E enterically transmitted. Hepatitis D, or delta hepatitis, is another distinct virus that is dependent upon hepatitis B infection. This form of hepatitis may occur as a super-infectionin a hepatitis B carrier or as a co-infection in an individual with acute hepatitis B. Hepatitis viruses most often found in the United States include A, B, C, and D. Because fatality from hepatitis is relatively low, mortality figures are a poor indicator of the actual incidence of these diseases. The Centers for Disease Control and Prevention estimated that approximately 400,000–600,000 people were infected with viral hepatitis during the decade of the 1990s. Hepatitis plagued mankind as early as the fifth century BC. It was referenced in early biblical literature and described as occurring in outbreaks, especially during times of war. Toward the end of the nineteenth century, hepatitis was thought to occur as a result of infection of the hepatic parenchyma. -

Localized to Vaccination Site (3-12-2003 Version) Low
Legend Tool 1 Morbidity and Mortality Risk Clinical Evaluation Tool for Smallpox Vaccine Adverse Reactions based on clinical presentation. Dermatologic Reactions/ Localized to Vaccination Site (3-12-2003 Version) Low www.bt.cdc.gov/agent/smallpox/vaccination/clineval Moderate NO YES Go to Dermatologic Reactions/ Nontoxic Appearance, Distant from High Close Contact of Vaccine Recipient? Vaccine Recipient? Vaccination Site (or in a Contact) Clinical Evaluation Tool. YES Erythematous Vaccination Normal Vaccination Reaction Severe Vaccination Reaction Reaction of Concern Typical reaction timeline Common signs/symptoms Tape sensitivity Rapid progressive painless extension of Day Description after vaccination Try different tape. Erythema present along lines of central vaccination lesion or progression YES 0 Vaccination - Pruritus Change bandage adhesive tape and no or mild without apparent healing after 15 days. 3-4 Papule - Soreness at frequently, rotate systemic symptoms? Lesion often necrotic. Initially little or no 5-6 Vesicle with surrounding vaccination site bandage, or take a inflammation. May present with few or no erythema - vesicle with - Intense erythema judicious bandage systemic symptoms. depressed center ringing the “holiday” 8-9 Well-formed pustule vaccination site remembering to use NO 12+ Pustule crusts over and - Small papules or other means (e.g. becomes a scab vesicles around long sleeves) to Early Progressive vaccinia (Vaccinia 17-21 Scab detaches revealing scar vaccination lesion prevent contact Erythema with induration, warmth, necrosum, Vaccinia gangrenosum) Timeline may be accelerated (satellite lesions) transmission. Use and pain. May also have regional Go to Clinical Evaluation Tool for in persons with history of prior - Headache antihistamines PRN; lymphadenopathy and fever. Dermatologic Reactions/Toxic smallpox vaccination. -

Abstract Book (PDF)
Abstract Book and Program 7th European Seminar in Virology (EuSeV) “Vaccines and antibodies against viral infections” June 14-16, 2019 Botanical Garden University of Padova 1 7th European Seminar in Virology (EuSeV) “Vaccines and antibodies against viral infections” June 14-16, 2019 Organizers: Gabriella Campadelli-Fiume, University of Bologna Dana Wolf, Hebrew University Jerusalem Michael Kann University of Gothenburg Thomas Mertens, Ulm University Medical Centre Giorgio Palù University of Padova Organizing Secretariat: Arianna Calistri, Ilaria Frasson, Michela Nandi, Marta Trevisan 2 7th. European Seminars in Virology (EuSeV) 2019 Program FRIDAY 14.06.2019 13:45-14:00 Welcome Dana Wolf, Gabriella Campadelli-Fiume, Thomas Mertens, Michael Kann Giorgio Palù Session on Ethical and technological issues about antiviral vaccine and antibodies development Chair: Giorgio Palù 14:00-14:40 Andrea Grignolio, Cognitive bases for vaccine hesitancy Medical Humanities & Bioethics, Vita-Salute San Raffaele University, Milan, Italy [email protected] 14:40-15:20 Rino Rappuoli, Reverse vaccinology 2.0 GSK, Siena, Italy [email protected] 15:20-16:00 Melvin Kohn, MSD’s Investigational Ebola Vaccine Regional Director of Medical Affairs Lead for Vaccines, MSD. [email protected] 16:00-16:30 Discussion 16:30-17:30 Selected oral presentations Francesco Santoro, Human transcriptomic response to vaccination with recombinant VSV expressing Ebola virus Glycoprotein Laboratory of Molecular Microbiology and Biotechnology (LAMMB), Dept. of Medical Biotechnologies, -

Current Opinion in Infectious Diseases Was Launched in 1988
June 2007, Volume 20, Issue 3,pp.237-344 Editorial introductions vii Editorial introductions. Paediatric and neonatal infections 237 Adverse events following immunization: perception and evidence. Jan Bonhoeffer; Ulrich Heininger 247 Managing congenital syphilis again? The more things change [horizontal ellipsis]. Rana Chakraborty; Suzanne Luck 253 Rotavirus vaccines in developed countries. Jim P Buttery; Carl Kirkwood 259 The burden of influenza in children. Mary Iskander; Robert Booy; Stephen Lambert 264 T cell-based diagnosis of childhood tuberculosis infection. Ajit Lalvani; Kerry A Millington 272 Aseptic meningitis. Bonita E Lee; H Dele Davies Pathogenesis and immune response 278 Herpesviral-bacterial synergy in the pathogenesis of human periodontitis. Jørgen Slots 284 Leptospirosis: pathogenesis, immunity, and diagnosis. Raghavan UM Palaniappan; Subbupoongothai Ramanujam; Yung-Fu Chang 293 Experimental infections with West Nile virus. Richard A Bowen; Nicole M Nemeth 298 The role of superantigens of group A Streptococcus and Staphylococcus aureus in Kawasaki disease. Kousaku Matsubara; Takashi Fukaya 304 Distinction between bacterial and viral infections. Jari Nuutila; Esa-Matti Lilius 311 New concepts in vaccine development in malaria. Bernard N Kanoi; Thomas G Egwang Current World Literature Bibliography 317 Current World Literature. Editorial introductions Current Opinion in Infectious Diseases was launched in 1988. Utah, Dr Stevens com- It is part of a successful series of review journals whose pleted an Infectious Dis- unique format is designed to provide a systematic and ease Fellowship at Brooke critical assessment of the literature as presented in the Army Medical Center in many primary journals. The field of infectious diseases is San Antonio, Texas, and divided into 12 sections that are reviewed once a year.