The Interaction of Laser Energy with Ureter Tissues in a Long Term Investigation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Reproductive System
27 The Reproductive System PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The reproductive system is designed to perpetuate the species • The male produces gametes called sperm cells • The female produces gametes called ova • The joining of a sperm cell and an ovum is fertilization • Fertilization results in the formation of a zygote © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • Overview of the Male Reproductive System • Testis • Epididymis • Ductus deferens • Ejaculatory duct • Spongy urethra (penile urethra) • Seminal gland • Prostate gland • Bulbo-urethral gland © 2012 Pearson Education, Inc. Figure 27.1 The Male Reproductive System, Part I Pubic symphysis Ureter Urinary bladder Prostatic urethra Seminal gland Membranous urethra Rectum Corpus cavernosum Prostate gland Corpus spongiosum Spongy urethra Ejaculatory duct Ductus deferens Penis Bulbo-urethral gland Epididymis Anus Testis External urethral orifice Scrotum Sigmoid colon (cut) Rectum Internal urethral orifice Rectus abdominis Prostatic urethra Urinary bladder Prostate gland Pubic symphysis Bristle within ejaculatory duct Membranous urethra Penis Spongy urethra Spongy urethra within corpus spongiosum Bulbospongiosus muscle Corpus cavernosum Ductus deferens Epididymis Scrotum Testis © 2012 Pearson Education, Inc. Anatomy of the Male Reproductive System • The Testes • Testes hang inside a pouch called the scrotum, which is on the outside of the body -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

The Digestive System
69 chapter four THE DIGESTIVE SYSTEM THE DIGESTIVE SYSTEM The digestive system is structurally divided into two main parts: a long, winding tube that carries food through its length, and a series of supportive organs outside of the tube. The long tube is called the gastrointestinal (GI) tract. The GI tract extends from the mouth to the anus, and consists of the mouth, or oral cavity, the pharynx, the esophagus, the stomach, the small intestine, and the large intes- tine. It is here that the functions of mechanical digestion, chemical digestion, absorption of nutrients and water, and release of solid waste material take place. The supportive organs that lie outside the GI tract are known as accessory organs, and include the teeth, salivary glands, liver, gallbladder, and pancreas. Because most organs of the digestive system lie within body cavities, you will perform a dissection procedure that exposes the cavities before you begin identifying individual organs. You will also observe the cavities and their associated membranes before proceeding with your study of the digestive system. EXPOSING THE BODY CAVITIES should feel like the wall of a stretched balloon. With your skinned cat on its dorsal side, examine the cutting lines shown in Figure 4.1 and plan 2. Extend the cut laterally in both direc- out your dissection. Note that the numbers tions, roughly 4 inches, still working with indicate the sequence of the cutting procedure. your scissors. Cut in a curved pattern as Palpate the long, bony sternum and the softer, shown in Figure 4.1, which follows the cartilaginous xiphoid process to find the ventral contour of the diaphragm. -

The Urinary System Dr
The urinary System Dr. Ali Ebneshahidi Functions of the Urinary System • Excretion – removal of waste material from the blood plasma and the disposal of this waste in the urine. • Elimination – removal of waste from other organ systems - from digestive system – undigested food, water, salt, ions, and drugs. + - from respiratory system – CO2,H , water, toxins. - from skin – water, NaCl, nitrogenous wastes (urea , uric acid, ammonia, creatinine). • Water balance -- kidney tubules regulate water reabsorption and urine concentration. • regulation of PH, volume, and composition of body fluids. • production of Erythropoietin for hematopoieseis, and renin for blood pressure regulation. Anatomy of the Urinary System Gross anatomy: • kidneys – a pair of bean – shaped organs located retroperitoneally, responsible for blood filtering and urine formation. • Renal capsule – a layer of fibrous connective tissue covering the kidneys. • Renal cortex – outer region of the kidneys where most nephrons is located. • Renal medulla – inner region of the kidneys where some nephrons is located, also where urine is collected to be excreted outward. • Renal calyx – duct – like sections of renal medulla for collecting urine from nephrons and direct urine into renal pelvis. • Renal pyramid – connective tissues in the renal medulla binding various structures together. • Renal pelvis – central urine collecting area of renal medulla. • Hilum (or hilus) – concave notch of kidneys where renal artery, renal vein, urethra, nerves, and lymphatic vessels converge. • Ureter – a tubule that transport urine (mainly by peristalsis) from the kidney to the urinary bladder. • Urinary bladder – a spherical storage organ that contains up to 400 ml of urine. • Urethra – a tubule that excretes urine out of the urinary bladder to the outside, through the urethral orifice. -
Laboratory 8 - Urinary and Reproductive Systems
Laboratory 8 - Urinary and Reproductive Systems Urinary System Please read before starting: It is easy to damage the structures of the reproductive system as you expose structures associated with excretion, so exercise caution as you do this. Please also note that we will have drawings available as well to help you find and identify the structures described below. The major blood vessels serving the kidneys are the Renal renal artery and the renal pyramid vein., which are located deep in the parietal peritoneum. The renal artery is a branch of the dorsal aorta that comes off Renal further caudal than the cranial pelvis mesenteric artery. Dissect the left kidney in situ, dividing it into dorsal and ventral portions by making a frontal section along the outer periphery. Observe the renal cortex renal medulla (next layer in) renal pyramids renal pelvis ureter (see above diagram) The kidneys include a variety of structures including an arterial supply, a venous return, extensive capillary networks around each nephron and then, of course, the filtration and reabsorption apparatus. These structures are primarily composed of nephrons (the basic functional unit of the kidney) and the ducts which carry urine away from the nephron (the collecting ducts and larger ducts eventually draining these into the ureters from each kidney. The renal pyramids contain the extensions of the nephrons into the renal medulla (the Loops of Henle) and the collecting ducts. Urine is eventually emptied into the renal pelvis before leaving the kidneys in the ureters. The ureters leaves the kidneys medially at approximately the midpoint of the organs and then run caudal to the urinary bladder. -

Urinary Retention
Urinary Retention National Kidney and Urologic Diseases Information Clearinghouse What is urinary retention? What is the urinary tract Urinary retention is the inability to and how does it work? empty the bladder completely. Urinary The urinary tract is the body’s drainage retention can be acute or chronic. Acute system for removing urine, which is urinary retention happens suddenly and composed of wastes and extra fluid. In lasts only a short time. People with acute order for normal urination to occur, all urinary retention cannot urinate at all, body parts in the urinary tract need to work even though they have a full bladder. together in the correct order. Acute urinary retention, a potentially life-threatening medical condition, Kidneys. The kidneys are two bean-shaped requires immediate emergency treatment. organs, each about the size of a fist. They Acute urinary retention can cause great are located just below the rib cage, one discomfort or pain. on each side of the spine. Every day, the kidneys filter about 120 to 150 quarts of Chronic urinary retention can be a long- blood to produce about 1 to 2 quarts of lasting medical condition. People with urine. The kidneys work around the clock; chronic urinary retention can urinate. a person does not control what they do. However, they do not completely empty all of the urine from their bladders. Ureters. Ureters are the thin tubes of Often people are not even aware they muscle—one on each side of the bladder— have this condition until they develop that carry urine from each of the kidneys to another problem, such as urinary the bladder. -

Ureter Urinary Bladder Seminal Vesicle Ampulla of Ductus Deferens
Ureter Urinary bladder Seminal vesicle Prostatic urethra Ampulla of Pubis ductus deferens Membranous urethra Ejaculatory duct Urogenital diaphragm Rectum Erectile tissue Prostate of the penis Bulbo-urethral gland Spongy urethra Shaft of the penis Ductus (vas) deferens Epididymis Glans penis Testis Prepuce Scrotum External urethral (a) orifice © 2018 Pearson Education, Inc. 1 Urinary bladder Ureter Ampulla of ductus deferens Seminal vesicle Ejaculatory Prostate duct Prostatic Bulbourethral urethra gland Membranous Ductus urethra deferens Root of penis Erectile tissues Epididymis Shaft (body) of penis Testis Spongy urethra Glans penis Prepuce External urethral (b) orifice © 2018 Pearson Education, Inc. 2 Spermatic cord Blood vessels and nerves Seminiferous tubule Rete testis Ductus (vas) deferens Lobule Septum Tunica Epididymis albuginea © 2018 Pearson Education, Inc. 3 Seminiferous tubule Basement membrane Spermatogonium 2n 2n Daughter cell (stem cell) type A (remains at basement Mitosis 2n membrane as a stem cell) Growth Daughter cell type B Enters (moves toward tubule prophase of lumen) meiosis I 2n Primary spermatocyte Meiosis I completed Meiosis n n Secondary spermatocytes Meiosis II n n n n Early spermatids n n n n Late spermatids Spermatogenesis Spermiogenesis Sperm n n n n Lumen of seminiferous tubule © 2018 Pearson Education, Inc. 4 Gametes (n = 23) n Egg n Sperm Meiosis Fertilization Multicellular adults Zygote 2n (2n = 46) (2n = 46) Mitosis and development © 2018 Pearson Education, Inc. 5 Provides genetic Provides instructions and a energy for means of penetrating mobility the follicle cell capsule and Plasma membrane oocyte membrane Neck Provides Tail for mobility Head Midpiece Axial filament Acrosome of tail Nucleus Mitochondria Proximal centriole (b) © 2018 Pearson Education, Inc. -
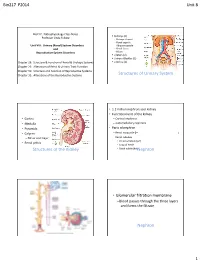
Structures of Urinary System
Bio217 F2014 Unit 8 Bio217: Pathophysiology Class Notes Kidneys (2) Professor Linda Falkow ◦ Retroperitoneal ◦ Renal capsule Unit VIII: Urinary (Renal) System Disorders ◦ Adipose capsule and ◦ Renal fascia Reproductive System Disorders ◦ Hilum Ureters (2) Urinary Bladder (1) Chapter 28: Structure & Function of Renal & Urologic Systems Urethra (1) Chapter 29: Alterations of Renal & Urinary Tract Function Chapter 31: Structure and Function of Reproductive Systems Chapter 32: Alterations of the Reproductive Systems Structures of Urinary System • 1.2 million nephrons per kidney • Functional unit of the kidney • Cortex – Cortical nephrons • Medulla – Juxtamedullary nephrons • Pyramids • Parts of nephron • Calyces – Renal corpuscle (=_______________________________) – Minor and major – Renal tubules • Proximal tubule (pct) • Renal pelvis • Loop of Henle Structures of the Kidney • Distal tubule (dct)Nephron • Glomerular filtration membrane –Blood passes through the three layers and forms the filtrate Nephron Nephron 1 Bio217 F2014 Unit 8 • Juxtaglomerular apparatus – Juxtaglomerular cells ( renin) – Macula densa (sense changes in Na+) – Renin-angiotensin pathway: ___________ • Decr. blood vol. or decr. Na+ incr. renin Angiotensin I Angiotensin II aldosterone (incr. reabsorption of Na+ and H2O) Nephron Juxtaglomerular Apparatus • Urinary Bladder – Detrusor muscle – Trigone – Micturition reflex • Urethra – Internal and external sphincters – 3 to 4 cm in females – 18 to 20 cm in males Structures of Urinary System Urinary Bladder and Urethra -

Day 6 Urogenital System
Day 6 Urogenital System: Urinary System The structures of the reproductive and urinary systems are often considered together as the urogenital systems, since they have common embryological origins. However, the emphasis in this dissection is on identifying the structures of the urinary tract with only a few references to contiguous reproductive structures. The anatomy of the reproductive system is studied in the next section. Identifying Organs of the Urinary System 1. Obtain your dissection specimen and place it on your dissection tray. Reflect the abdominal viscera (most importantly the small intestine) to locate the kidneys high on the dorsal body wall. Note that the kidneys in the cat, as well as in the human are retroperitoneal (behind the peritoneum). 2. Carefully remove the peritoneum, and clear away the bed of fat that invests the kidneys. Then locate the adrenal (suprarenal) glands that lie superiorly and medial to the kidneys. 3. Identify the renal artery (red latex injected), the renal vein (blue latex injected), and the ureter at the hilus region of the kidney. (You may find two renal veins leaving one kidney in the cat but not in humans). 4. Trace the ureters to the urinary bladder, a smooth muscular sac located superiorly to the small intestine. If your cat is a female, be careful not to confuse the ureters with the urine tubes, which lie superior to the bladder in the same general region. See Figure D8.1. Observe the sites where the ureters enter the bladder. How would you describe the entrance point anatomically? 5. Cut through the bladder wall, and examine the region of the uretheral exit to see if you can discern any evidence of the internal sphincter. -

Fetal Pig Dissection What Do You Think Humans Have in Common with the Pig?
Fetal Pig Dissection What do you think humans have in common with the pig? https://ferrebeekeeper.files.wordpress.com/2014/03/farmer-clip-art-4.gif http://www.clipartpanda.com/categories/pig-in-mud-cartoon Humans and Pigs may be closer than you think! Both are mammals We share common body systems The anatomy of the pig is close to that of humans The fetal pigs will tell us more about our own bodies and give us a way to explore! http://www.fanpop.com/clubs/human-anatomy/images/10358267/title/human-anatomy-photo http://www.biologycorner.com/pig/fetal_pig02.jpg SAFETY FIRST! ❖ NEVER point sharp ❖ Wash hand at the objects at yourself or end of each class others period ❖ Always cut/make ❖ Don’t remove any incisions away form specimens from the yourself and others classroom ❖ No horseplay in the ❖ Properly dispose of lab any materials ❖ Properly mount ❖ Clean up area specimens to the dissection pan ❑ Descriptive words are used to describe “where” on an animal. ❑ Like using North, South, East, or West for locations on a map. Dorsal -- toward the back Ventral -- toward the front/belly Separated by the frontal plane V Cranial -- toward the head Rostral -- toward the nose/beak Caudal -- toward the tail Separated by the transverse plane Medial – directed toward the midline (sagittal plane) Lateral -- directed away from the midline (sagittal plane) Sagittal Plane Proximal -- located close to the sagittal line of the body. Distal -- located away from the sagittal line of the body External Anatomy ❖ Skin ❖ Nose ❖ Tongue ❖ Eyelids ❖ External Ear ❖ Digits ❖ Umbilical Cord ❖ Identify the Sex ❖ 2 umbilical arteries ❖ Male – Scrotal Sac (ventral to anus) ❖ Umbilical vein and Urogenital Opening ❖ Teats ❖ Female – Urogenital opening (ventral ❖ Anus to anus) and genital papilla DEMO SLIDE BOX 23 Commercial slide. -

A Rare Cause of Ureteric Stricture and Hydronephrosis: Metastatic Esophageal Cancer to the Urinary Bladder
Goh et al. Mini-invasive Surg 2019;3:17 Mini-invasive Surgery DOI: 10.20517/2574-1225.2019.06 Case Report Open Access A rare cause of ureteric stricture and hydronephrosis: metastatic esophageal cancer to the urinary bladder Darren Goh, Xin Ling Teo, Sey Kiat Terence Lim Department of Urology, Changi General Hospital, Singapore 529889, Singapore. Correspondence to: Dr. Darren Goh, Department of Urology, Changi General Hospital, 2 Simei Street 3, Singapore 529889, Singapore. E-mail: [email protected] How to cite this article: Goh D, Teo XL, Lim SKT. A rare cause of ureteric stricture and hydronephrosis: metastatic esophageal cancer to the urinary bladder. Mini-invasive Surg 2019;3:17. http://dx.doi.org/10.20517/2574-1225.2019.06 Received: 12 Feb 2019 First Decision: 4 May 2019 Revised: 15 May 2019 Accepted: 17 May 2019 Published: 18 Jun 2019 Science Editor: Giulio Belli Copy Editor: Cai-Hong Wang Production Editor: Huan-Liang Wu Abstract The presence of hydronephrosis usually signifies the presence of significant urinary tract obstruction, more commonly at the level of the ureter, and occasionally at the bladder outlet in cases of bilateral hydronephrosis. Unilateral hydronephrosis is most commonly caused by a ureteric stone or stricture, and rarely caused by neoplasm. Metastatic disease to the urinary bladder is rare and usually presents with hematuria, and we report the first case of hydronephrosis resulting from a metastatic esophageal cancer to the bladder. Keywords: Hydronephrosis, metastatic esophageal cancer, ureteric stricture INTRODUCTION Unilateral hydronephrosis is most commonly caused by obstruction of the ureter due to the presence of a ureteric stone or stricture, and rarely secondary to a primary ureteric or bladder neoplasm or from direct invasion or external compression by locally advanced cancers from adjacent organs such as the lower gastrointestinal and female genitourinary tract. -

Development of the Bladder and Ureterovesical Junction
Differentiation xxx (xxxx) xxx–xxx Contents lists available at ScienceDirect Differentiation journal homepage: www.elsevier.com/locate/diff ☆ Development of the human bladder and ureterovesical junction ⁎ Aron Liaw, Gerald R. Cunha, Joel Shen, Mei Cao, Ge Liu, Adriane Sinclair, Laurence Baskin Department of Urology, University of California, San Francisco, San Francisco, CA Division of Pediatric Urology, University of California San Francisco Benioff Children's Hospital, San Francisco, CA 94143, United States ARTICLE INFO ABSTRACT Keywords: The urinary bladder collects urine from the kidneys and stores it until the appropriate moment for voiding. The Bladder trigone and ureterovesical junctions are key to bladder function, by allowing one-way passage of urine into the Fetal bladder without obstruction. Embryological development of these structures has been studied in multiple animal Development. Trigone, ureterovesical junction models as well as humans. In this report we review the existing literature on bladder development and cellular signalling with particular focus on bladder development in humans. The bladder and ureterovesical junction form primarily during the fourth to eighth weeks of gestation, and arise from the primitive urogenital sinus following subdivision of the cloaca. The bladder develops through mesenchymal-epithelial interactions between the endoderm of the urogenital sinus and mesodermal me- senchyme. Key signalling factors in bladder development include shh, TGF-β, Bmp4, and Fgfr2. A concentration gradient of shh is particularly important in development of bladder musculature, which is vital to bladder function. The ureterovesical junction forms from the interaction between the Wolffian duct and the bladder. The ureteric bud arises from the Wolffian duct and is incorporated into the developing bladder at the trigone.