Ground Water Quality Status with Respect to Fluoride Contamination
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List-Of-TO-STO-20200707191409.Pdf
Annual Review Report for the year 2018-19 Annexure 1.1 List of DTOs/ATOs/STOs in Andhra Pradesh (As referred to in para 1.1) Srikakulam District Vizianagaram District 1 DTO, Srikakulam 1 DTO, Vizianagaram 2 STO, Narasannapeta 2 STO, Bobbili 3 STO, Palakonda 3 STO, Gajapathinagaram 4 STO, Palasa 4 STO, Parvathipuram 5 STO, Ponduru 5 STO, Salur 6 STO, Rajam 6 STO, Srungavarapukota 7 STO, Sompeta 7 STO, Bhogapuram 8 STO, Tekkali 8 STO, Cheepurupalli 9 STO, Amudalavalasa 9 STO, Kothavalasa 10 STO, Itchapuram 10 STO, Kurupam 11 STO, Kotabommali 11 STO, Nellimarla 12 STO, Hiramandalam at Kothur 12 STO, Badangi at Therlam 13 STO, Pathapatnam 13 STO, Vizianagaram 14 STO, Srikakulam East Godavari District 15 STO, Ranasthalam 1 DTO, East Godavari Visakhapatnam District 2 STO, Alamuru 1 DTO, Visakhapatnam 3 STO, Amalapuram 2 STO, Anakapallli (E) 4 STO, Kakinada 3 STO, Bheemunipatnam 5 STO, Kothapeta 4 STO, Chodavaram 6 STO, Peddapuram 5 STO, Elamanchili 7 DTO, Rajahmundry 6 STO, Narsipatnam 8 STO, R.C.Puram 7 STO, Paderu 9 STO, Rampachodavaram 8 STO, Visakhapatnam 10 STO, Rayavaram 9 STO, Anakapalli(W) 11 STO, Razole 10 STO, Araku 12 STO, Addateegala 11 STO, Chintapalli 13 STO, Mummidivaram 12 STO, Kota Uratla 14 STO, Pithapuram 13 STO, Madugula 15 STO, Prathipadu 14 STO, Nakkapalli at Payakaraopeta 16 STO, Tuni West Godavari District 17 STO, Jaggampeta 1 DTO, West Godavari 18 STO, Korukonda 2 STO, Bhimavaram 19 STO, Anaparthy 3 STO, Chintalapudi 20 STO, Chintoor 4 STO, Gopalapuram Prakasam District 5 STO, Kovvur 1 ATO, Kandukuru 6 STO, Narasapuram -

1. EXECUTIVE SUMMARY 1.1 Introduction the Proposed Mining Lease Area for Black Granite Over an Extent of 5.0Hectares Is Located in Sy
Black Granite of Sri. S. Balakrishna Pre-Feasibility Report 1. EXECUTIVE SUMMARY 1.1 Introduction The proposed mining lease area for Black Granite over an extent of 5.0Hectares is located in Sy. No. 51/P of of Ummadivaram Village, Vinukonda Mandal, Guntur District, Andhra Pradesh. The lease was granted by Asst. Director of Mines & Geology, Dachepalli vide proceddings No. 3447/Q/2005 dated 02.12.2009 for 20 years for Black Granite in favour of Sri.S.Balakrishna. The Mining Scheme was approved by Deputy Director of Mines and Geology, Guntur vide letter No.2582/MS/GNT/2015, dated 01.07.2016. The salient features of the project are given Table 1.1. TABLE 1.1: Salient features of the project Project Name Proposed Black Granite Mine of Sri. S.Balakrishna Mining Lease Area 5.0Hectares Sy. No. 51/P of of Ummadivaram Village, Vinukonda Location Mandal, Guntur District, Andhra Pradesh Toposheet No. 57 J/4 Topography The lease area is a hilly Terrain. Minerals of mine Black Granite Proposed production of mine. 6000 Cu. m/ Annum Method of mining open cast Semi-mechanization method No. of working days 300 days Water demand 12KLD Sources of water Bore well & Tankers Ultimate depth of Mining 6 m Man power 12 Nearest railway station Vinukonda railway station is about 13.78 km due NW Nearest Airport Vijayawada Airport is about 116.5 km Project Cost Rs. 50 Lakhs 2. INTRODUCTION OF THE PROJECT/ BACKGROUND INFORMATION 2.1 Identification of Project and Project Proponent Sri S. Balakrishna is a private Lessee in granite business. -

Assessment and Comparison of Ground Water Quality in Guntur District, Andhra Pradesh by Using ARC GIS
Volume 3, Issue 4, April – 2018 International Journal of Innovative Science and Research Technology ISSN No:-2456-2165 Assessment and Comparison of Ground water Quality in Guntur District, Andhra Pradesh by using ARC GIS K.T.V Subba Rao1, D.Venkatesh1, S.Farooq Ahmed2 1B.Tech Student, Department of civil Engineering, VVIT, Nambur, Guntur, India 2Associate Professor, Department of civil Engineering, VVIT, Nambur, Guntur, India Abstract:- Groundwater normally clean and free of bacteria as it is filtered through various soil layers. But due How well loosely arranged rock (such as sand and to Rapid urbanization, industrialization and improper gravel) holds water depends on the size and shape of the rock disposal of waste that polluting even groundwater. particles. Layers of loosely arranged particles of uniform size Objective of this study is to assess and map the spatial tend to hold more water than layers of rock with materials of distribution of groundwater quality in Guntur District different sizes. This is because smaller rock materials can Andhra Pradesh, using geographical information system relax in space between larger rocks, decreasing the space (GIS). As the capital region is laid in between Guntur and available to hold water. It is usually much cleaner than surface Krishna district. Groundwater is the main source for water. Source of ground water is rain and snow that falls to the industrialization and urbanization. Here the physical and ground a portion of which percolates down into the ground to chemical properties analytical data of groundwater sample become ground water.25-40% of world population is using data of 30 well points of various regions in Guntur district groundwater for drinking purpose. -

Handbook of Statistics Guntur District 2015 Andhra Pradesh.Pdf
Sri. Kantilal Dande, I.A.S., District Collector & Magistrate, Guntur. PREFACE I am glad that the Hand Book of Statistics of Guntur District for the year 2014-15 is being released. In view of the rapid socio-economic development and progress being made at macro and micro levels the need for maintaining a Basic Information System and statistical infrastructure is very much essential. As such the present Hand Book gives the statistics on various aspects of socio-economic development under various sectors in the District. I hope this book will serve as a useful source of information for the Public, Administrators, Planners, Bankers, NGOs, Development Agencies and Research scholars for information and implementation of various developmental programmes, projects & schemes in the district. The data incorporated in this book has been collected from various Central / State Government Departments, Public Sector undertakings, Corporations and other agencies. I express my deep gratitude to all the officers of the concerned agencies in furnishing the data for this publication. I appreciate the efforts made by Chief Planning Officer and his staff for the excellent work done by them in bringing out this publication. Any suggestion for further improvement of this publication is most welcome. GUNTUR DISTRICT COLLECTOR Date: - 01-2016 GUNTUR DISTRICT HAND BOOK OF STATISTICS – 2015 CONTENTS Table No. ItemPage No. A. Salient Features of the District (1 to 2) i - ii A-1 Places of Tourist Importance iii B. Comparision of the District with the State 2012-13 iv-viii C. Administrative Divisions in the District – 2014 ix C-1 Municipal Information in the District-2014-15 x D. -

Mandal Special Officers Details in Guntur District As on 07.10.2019
MANDAL SPECIAL OFFICERS DETAILS IN GUNTUR DISTRICT AS ON 07.10.2019 Sl. Name of the Name of the Officer Mobile No Designation of the Officer No. Mandal 1 AMARAVATHI Sri G. VEERAIAH 9849903376 DIVISIONAL PANCHYAT OFFICER, GUNTUR CHOUDARI 2 ATCHAMPET Sri P. RAJESH BABU 9100109186 DISTRICT CO - OPERATIVE AUDIT OFFICER, GUNTUR 3 BELLAMKONDA Smt. K. AMALA KUMARI 8886614114 ASSISTANT DIRECTOR OF AGRICULTURE, KROSURU 4 GUNTUR RURAL Smt. C. PADMAVATHI 8886614118 ASSISTANT DIRECTOR OF AGRICULTURE, O/o @ Piduguralla. 5 KROSURU Sri.Y. AJAY KUMAR 9640909823 GENERAL MANAGER, DISTRICT INDUSTRIES CENTER, GUNTUR. 6 MANGALAGIRI Smt. Y.V. PRASANNA 9440814511 PROJECT DIRECTOR, DW & CDA, GUNTUR. LAKSHMI 7 MEDIKONDURU Smt. M. VARALAKSHMI 9182361247 ASSISTANT DIRECTOR, MARKETING, GUNTUR 8 MUPPALLA Sri CH. RAVI KUMAR 8886614107 ASSISTANT. DIRECTOR, AGRICULTURE (R) – SATTENAPALLI 9 PEDAKAKANI Sri T. SRINIVASA RAO 8886614142 ASSISTANT DIRECTOR, AGRICULTURE, PESTISIDES, GUNTUR. DEPUTY EXECUTIVE ENGINEER, PIU, SUB 10 PEDAKURAPADU Sri B.RAMA RAO 9849086958 DIVISION, PEDAKURAPADU@ SATTENAPALLI 11 PEDANANDIPADU Sri.SRINIVASARAO 8886614123 ASSISTANT. DIRECTOR, AGRICULTURE, GUNTUR 12 PHIRANGIPURAM Smt. RAJESWARI 7702003536 DISTRICT MANAGER CIVIL SUPPLIES, GUNTUR 13 PRATHIPADU Sri B.SAMBAIAH 9849389962 DEPUTY EXECUTIVE ENGINEER, PRI SUB DIVISION, PRATHIPADU@GUNTUR 14 RAJUPALEM Smt. RAMA DEVI 7995552871 DISTRICT SOCIAL WELFARE OFFICER, GUNTUR 15 SATTENAPALLI 9100109189 DIVISIONAL CO - OPERATIVE OFFICER, Sri.D. SRINIVASARAO NARASARAOPET. 16 TADEPALLI Smt P. MASTANAMMA 8886614116 ASSISTANT DIRECTOR, AGRICULTURE, MANGALAGIRI 17 TADIKONDA Sri SAYYAD RAFI AHAMED 9182361228 ASSISTANT DIRECTOR OF MARKETING, AMC TADIKONDA. 18 THULLURU Smt. KALPANA 9963899994 DISTRICT B.C.WELFARE OFFICER, GUNTUR 19 VATTICHERUKURU Smt.D. DURGA BAI 7702057456 DISTRICT EMPLOYMENT OFFICER, GUNTUR 20 BOLLAPALLI Sri. T. SUBBARAO 8886614148 MANDAL AGRICULTURE OFFICER, IPURU. -

B.Ed. Colleges: GUNTUR DISTRICT L
B.Ed. Colleges: GUNTUR DISTRICT l. Sanviya College of Education, Bapatla-5221 0 1 2. St.Peter & GK College of Education, Burripalem-522 301, Tenali 3. Nagarjuna College of Education, Kavur (Md), Cherukupalli-522 309 4. AMG College of Education, G.T. Rd., Chilakaluripet-522 616 5. Acharya N.G.Ranga College of Education,Chilumuru-522 301 6. Sri Venkateswara Balakuteer College of Education, Chowdavaram-522 019 7. Palnadu College of Education, Narayanapuram,Dachepalli-522 414 8. AL College of Education, Lodge Centre, Guntur-522 002 9. AM College of Education, Ponnur Road, Guntur-522 003 10. Bhashyam College of Education, Gorantla, Guntur-522 034 1l. GVR & S College of Education, Ring Rd., Chandramoulinagar, Guntur-522 006 12. Sweekar College of Special Education (M.R.), Amaravathi Rd., Guntur 13. SMS & SMCMR College of Education, Syamalanagar, Guntur-522 006. 14. Coramandal College of Education, Medikonduru, Guntur Dt 15. Vyshnavi College of Education, Jayanthinagar, Gurazala-522 415 16. HMKS & MGSM College of Education, Kanagala-522 259, Guntur dt. 17. Sri Venkateswara College of Education, Kollipara-522 304 18. Aman Showkath B.Ed. College, Issapalem-522 601, Narasaraopet (M) 19. St. Mark NTR College of Education, Peddacheruvu, Narasaraopet-522 601 20. AM Reddy College of Education, Mastan Reddy Nagar, Petlurivaripalem, 21. Lakshmi Narasimha B.Ed. College, Paturu, Narasaraopet-522 601. 22. Dr. Zakir Hussain College of Education, Mulukuduru, Ponnur. 23. Geethika College of Education, Ramireddypet, Piduguralla-522413 24. Repalle Christian College of Education, Gospel Fields, Repalle-522 265 25. SNBT Memorial College of Education for Women, Repalle-522 265 26. Nalanda College of Education 27. -

Guntur District
Guntur District S.No. Name of the Health care facility 1. The Superintendent, Government General Hospital, Guntur. Guntur District. 2. The Medical Officer, Yarlagadda Venkanna Chowdary Oncology Wing & Research Centre, Chinakakani (V), Guntur Rural (M), Guntur District. 3. All India Institute of Medical Sciences (AIIMS), Mangalagiri (V&M), Guntur District 4. The Medical Superintendent, District Hospital, Tenali (V&M), Guntur District 5. The Medical Officer, Community Health Centre , Chilakaluripet (V& M), Guntur District. 6. The Medical Officer, Area Hospital, Bapatla (V&M), Guntur District. 7. The Medical Superintandant, Area Hospital, Narasaraopet (V&M), Guntur District 8. Mukhyamantri Arogya Kendram (e -UPHC ), 5/1, Tuffan Nagar, Hanumayya Company Backside, Maruthi Nagar, Guntur, Guntur District 9. Mukhyamantri Arogya Kendram (e -UPHC ),Opp: ITC, 60 Feet Road, Behind Water Tank, Srinivasa Rao Thota, Guntur, Guntur District 10. Mukhyamantri Arogya Kendram (e -UPHC ), 3rd lane, Anandpet, Guntur, Guntur District. 11. Mukhyamantri Arogya Kendram (e -UPHC ),Venugopal Nagar, 8th lane, N.G.O. Colony, Guntur, Guntur District 12. Mukhyamantri Arogya Kendram (e -UPHC ), Mangaladas Nagar, 2nd Lane, Guntur Guntur District. 13. Mukhyamantri Arogya Kendram (e -UPHC ), Mallikarjunapeta Ratnapuri Colony, Beside Ambedkar Statue, Guntur, Guntur District. 14. Mukhyamantri Arogya Kendram (e -UPHC ),Opp: Vijaya Lakshmi Theather, Gundarao Pet, Beside Darga, Nallapadu Road, Guntur, Guntur District. 15. Mukhyamantri Arogya Kendram (e -UPHC ), 5/2, IPD Colony, Beside Sai Baba Temple, Ponnur Road, Guntur, Guntur District. 16. Mukhyamantri Arogya Kendram (e -UPHC ), L.B. Nagar, Beside Water Tank, Mani Hotel Centre, Guntur, Guntur District 17. Mukhyamantri Arogya Kendram (e -UPHC ), Lanchester Ro ad, Beside Ice Factory, Sangadigunta, Guntur, Guntur District. -
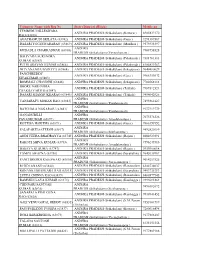
Volunteer Name with Reg No State (District)
Volunteer Name with Reg No State (District) (Block) Mobile no TEMBURU DILLESWARA ANDHRA PRADESH (Srikakulam) (Kotturu ) 8985832170 RAO (63838) AGATHAMUDI SRILATA (63742) ANDHRA PRADESH (Srikakulam) (Gara ) 9291389567 DASARI YOGESWARARAO (63869) ANDHRA PRADESH (Srikakulam) (Mandasa ) 9573933397 ANDHRA MUDADLA CHAKRADHAR (63868) 9908758528 PRADESH (Srikakulam) (Veeraghattam ) BALIVADA SURENDRA ANDHRA PRADESH (Srikakulam) (Palakonda ) 9381761383 KUMAR (63867) PUTHI SRAVAN KUMAR (63866) ANDHRA PRADESH (Srikakulam) (Palakonda ) 8186829362 NITYANANDA BADITYA (63864) ANDHRA PRADESH (Srikakulam) (Ichapuram) 9640480629 PANCHIREDDY ANDHRA PRADESH (Srikakulam) (Gara ) 9966353032 SIVAKUMAR (63863) BOMMALI CHANDINI (63848) ANDHRA PRADESH (Srikakulam) (Ichapuram) 7702668113 BOORE NARENDRA ANDHRA PRADESH (Srikakulam) (Tekkali) 9989512521 CHAKRAVARTHI (63847) DASARI SHANMUKHARAO (63846) ANDHRA PRADESH (Srikakulam) (Tekkali) 9494042926 ANDHRA VANJARAPU MOHAN RAO (63843) 7893862127 PRADESH (Srikakulam) (Kotabommali) ANDHRA BATCHALA NAGARAJU (63841) 9177212779 PRADESH (Srikakulam) (Kotabommali) GANADUBILLI ANDHRA 7075576526 PAVANKUMAR (63697) PRADESH (Srikakulam) (Amadalavalasa ) KOTTISA HARITHA (63871) ANDHRA PRADESH (Srikakulam) (Gara ) 9966153952 ANDHRA PALAPARTI SATEESH (63817) 9492820189 PRADESH (Srikakulam) (Seethampeta ) ANDI VEERA BHADRAYYA (63745) ANDHRA PRADESH (Srikakulam) (Rajam ) 8008929291 ANDHRA RAKOTI SHIVA KUMAR (63733) 7396243916 PRADESH (Srikakulam) (Amadalavalasa ) BASAVA KUSUMA (63747) ANDHRA PRADESH (Srikakulam) (Rajam ) 9959900618 -

Territorial Jurisdiction of Civil Court in Guntur District
TERRITORIAL JURISDICTION OF CIVIL COURT IN GUNTUR DISTRICT Sl. Name of the Court Names of Mandals No. Revenue Mandals of Guntur DMC: 1. Guntur 2. Pedakakani 3. Prathipadu 4. Vatticherukuru 5. Chebrolu Mangalagiri DMC: 1. Mangalagiri 2. Tadepalli 3. Tadikonda 4. Thulluru Bapatla: 1. Bapatla 2. Karlapalem 3. Pittalavanipalem 01 Prl. Sessions Court, Guntur 4. Pedanandipadu Ponnur DMC:- 1. Ponnuru 2. Kakumanu Sattenapalli DMC: 1. Sattenapalli 2. Bellamkonda 3. Rajupalem 4. Krosuru 5. muppalla 6. Atchempet 7. Pedakurapadu 8. Phirangipuram 9. Medikonduru 10. Amaravathi ---- and designated for Land Grabbing Cases Revenue Mandals of Guntur DMC: 1. Guntur 2. Pedakakani 3. Prathipadu 02 I Addl. Sessions Court, Guntur 4. Vatticherukuru 5. Chebrolu Mangalagiri DMC: 1. Mangalagiri 2. Tadepalli TERRITORIAL JURISDICTION OF CIVIL COURT IN GUNTUR DISTRICT 3. Tadikonda 4. Thulluru Bapatla: 1. Bapatla 2. Karlapalem 3. Pittalavanipalem 4. Pedanandipadu Ponnur DMC:- 1. Ponnuru 2. Kakumanu Sattenapalli DMC: 1. Sattenapalli 2. Bellamkonda 3. Rajupalem 4. Krosuru 5. muppalla 6. Atchempet 7. Pedakurapadu 8. Phirangipuram 9. Medikonduru 10. Amaravathi 03 II Addl. District Court, Guntur Revenue Mandals of Guntur DMC: 1. Guntur 2. Pedakakani 3. Prathipadu 4. Vatticherukuru 5. Chebrolu Mangalagiri DMC: 1. Mangalagiri 2. Tadepalli 3. Tadikonda 4. Thulluru Bapatla: 1. Bapatla 2. Karlapalem 3. Pittalavanipalem 4. Pedanandipadu Ponnur DMC:- 1. Ponnuru 2. Kakumanu Sattenapalli DMC: 1. Sattenapalli 2. Bellamkonda 3. Rajupalem 4. Krosuru 5. muppalla TERRITORIAL JURISDICTION OF CIVIL COURT IN GUNTUR DISTRICT 6. Atchempet 7. Pedakurapadu 8. Phirangipuram 9. Medikonduru 10. Amaravathi Revenue Mandals of Guntur DMC: 1. Guntur 2. Pedakakani 3. Prathipadu 4. Vatticherukuru 5. Chebrolu Mangalagiri DMC: 1. -

State District Branch Address Centre Ifsc
STATE DISTRICT BRANCH ADDRESS CENTRE IFSC CONTACT1 CONTACT2 CONTACT3 MICR_CODE A.N.REDDY NAGAR ANDHRA A N REDDY BR,NIRMAL,ANDHRA PRADESH ADILABAD NAGAR PRADESH NIRMAL ANDB0001972 8734243159 NONMICR 3-2-29/18D, 1ST CH.NAGAB FLOOR, AMBEDKAR HUSHANA ANDHRA CHOWK ADILABAD - M 08732- PRADESH ADILABAD ADILABAD 504 001 ADILABAD ANDB0000022 230766 TARA COMPLEX,MAIN ANDHRA ROAD,ASIFABAD,ADI 08733 PRADESH ADILABAD ASIFABAD LABAD DT - 504293 ASIFABAD ANDB0002010 279211 504011293 TEMPLE STREET, BASARA ADILABAD, ANDHRA ADILABAD, ANDHRA 986613998 PRADESH ADILABAD BASARA PRADESH-504104 BASAR ANDB0001485 1 Bazar Area, Bellampally , Adilabad G.Jeevan Reddy ANDHRA Dist - - 08735- PRADESH ADILABAD Bellampalli Bellampalli ADILABAD ANDB0000068 504251 2222115 ANDHRA BANK, BHAINSA BASAR P.SATYAN ROAD BHAINSA- ARAYANA - ANDHRA 504103 ADILABAD 08752- PRADESH ADILABAD BHAINSA DIST BHAINSA ANDB0000067 231108 D.NO 4-113/3/2,GOVT JUNIOR COLLEGE ROAD,NEAR BUS ANDHRA STAND,BOATH - 949452190 PRADESH ADILABAD BOATH 504305 BOATH ANDB0002091 1 MAIN ROAD,CHENNUR, ADILABAD DIST, ANDHRA CHENNUR, ANDHRA 087372412 PRADESH ADILABAD CHENNUR PRADESH-504201 CHINNOR ANDB0000098 36 9-25/1 BESIDE TANISHA GARDENS, ANDHRA DASNAPUR, PRADESH ADILABAD DASNAPUR ADILABAD - 504001 ADILABAD ANDB0001971 NO NONMICR ORIENT CEMENT WORKS CO, DEVAPUR,ADILABAD DIST, DEVAPUR, ANDHRA ANDHRA PRADESH- 08736 PRADESH ADILABAD DEVAPUR 504218 DEVAPUR ANDB0000135 240531 DOWEDPALLI, LXXETTIPET 08739- ANDHRA VILLAGE, GANDHI DOWDEPAL 233666/238 PRADESH ADILABAD DOWDEPALLI CHOWK LI ANDB0000767 222 H NO 1-171 VILL -

Guntur Division - Jurisdiction Map Bommaipalli ( BMMP ) 8.60
To Kazipet South Central Railway Border at Km. 4.50 Pagidipalli ( PGDP ) 4.02 Guntur Division - Jurisdiction map Bommaipalli ( BMMP ) 8.60 Nagireddipalli ( NRDP ) 19.44 Reference 1. Sr.DEN/North/GNT Jurisdiction Valigonda ( VLG ) 29.47 a. ADEN/North/GNT Jurisdiction Ramannapet ( RMNP ) 42.12 Nalgonda b. ADEN/NLDA Jurisdiction Chityala ( CTYL ) 51.08 Telangana 2. DEN/West/GNT Jurisdiction Srirampuram ( SRMR ) 66.31 c. ADEN/West/GNT Jurisdiction d. ADEN/NDL Jurisdiction Nalgonda ( NLDA ) 76.71 3. Double line Thipparthi ( TPPI ) 94.11 Krishna River crossing @ Km. 138.75 Miryalaguda (MRGA)114.40 Janpahad(JNPD)(10.64) Kodrapol ( KDGL ) 125.92 Vishnupuram (VNUP) 134.97 Border at km. 25.36 Pondugala (PDGL) 143.78 Mangalagiri (MAG) 20.28 Piduguralla (PGRL) 74.10 Bellamkonda (BMKD) 60.42 Tummalacheruvu (TMLU) 83.34 Reddigudem (REM) 54.29 Namburu (NBR) 8.97 Vijayawada Jn..(BZA) Nadikude Jn.(NDKD) 152.69/95.25 Pedakakani (PDKN) 6.13 Gurajala (GZA) 106.98 Sattenapalli (SAP) 42.37 Pedakurapadu (PKPU) 29.64 Reddipalem (REP) 2.47 Rentachintala (RCA) 115.16 Nudurupadu (NDPU)Gudipudi (GPDE) 35.71 28.19 Lingamguntla (LIM) 26.56 Krishna canal Jn..(KCC) 423.71 Phirangipuram (PPM) 21.07Siripuram (SRPM) 22.96 Macherla (MCLA) 130.26 Narasaraopet (NRT) 45.30 Mandapadu Halt (MDPD) 18.84 Bandarupalli (BDPL) 13.58 Satulur (STUR) 34.29Perecherla (PRCA) 11.26 Mahabubnagar Cheekateegalapalem ( CEM ) Munumaka89.55 (MUK) 52.99 Nallapadu (NLPD) 5.00 Gundlakamma (GKM) 95.85 Tenali Jn.(TEL) 397.25 Chinaravuru (CIV) 2.91 Guntur Zampani (ZPI) 9.80 Kurichedu (KCD) 107.27 Vemuru (VMU) 13.77 Potlapadu (POO) 113.85 Jaggam Bhotla Krishnapuram (JBK) 176.94 Donakonda (DKD) 120.08 Gajjalakonda (GJJ) 130.78 Markapur Rd. -

Media Scanning & Verification Cell
Media Scanning & Verification Cell Media alert from the Media Scanning & Verification Cell, IDSP-NCDC. Publication Reporting Alert ID Place Name News Source/Publication Language Date Date www.newindianexpress.com/English Guntur 6255 13.08.2021 17.08.2021 https://www.newindianexpress.com/states/andhra- Andhra Pradesh pradesh/2021/aug/13/60-fall-ill-in-kotcherla-medical- camp-set-up-2344117.html 60 fell ill in Guntur's Kotcherla, medical camp set up, Title: Andhra Pradesh Action By CSU, IDSP Information communicated to DSU- Guntur, SSU- Andhra Pradesh –NCDC About 60 people of Kotcherla village in Ipur mandal in Narasaraopet division were feeling unwell for the past 15 days. As all villagers are suffering from similar symptoms such as fever, cough and splitting headache, the district administration has set up a health camp and conducted Covid-19, typhoid and dengue tests out of which seven have tested positive for typhoid and three for dengue. The roads and the areas have been sanitised and drinking water samples have also been tested for contamination. District medical and health officer (DM&HO) Dr Yasmin, local MLA Bolla Brahmanaidu visited the village and inspected the special medical camp. People suffering from high fever have been shifted to Vinukonda, Narasaraopet, and Guntur GGH for better treatment. The DM&HO said the action will be taken on the officials who failed to assess the situation and take swift action. Save Water- Save Life, Save a tree- Don't print unless it's really necessary! Disclaimer:- This is a media alert subject