Quality Characteristics of Soy and Soy-Wheat Tempeh
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Textured Soy Protein (TSP)
PREMIER FOOD AND MACHINERY.CO.LTD 68/76-77 Moo5 Rama2Rd., Jomthong Dist, Bangkok 10150 Thailand Tel (66-2)476-6901, 477-1045, 476-9690, 877-0525-7 Textured Soy Protein (TSP) Textured or texturized vegetable protein (TVP), also known as Textured Soy Protein (TSP), soy meat is a defatted soy flour product, a by-product of extracting soybean oil. It is often used as a meat analogue or meat extender. It is quick to cook, with a protein content comparable to certain meats. TVP can be made from soy flour or concentrate, containing 50% and 70% soy protein, respectively; they have a mild beany flavor. Both require rehydration before use, sometimes with flavoring added in the same step. TVP is extruded, causing a change in the structure of the soy protein which results in a fibrous, spongy matrix, similar in texture to meat. In its dehydrated form, TVP has a shelf life of longer than a year, but will spoil within several days after being hydrated. In its flaked form, it can be used similarly to ground meat. Tel. 02-476-6901, 081-6235918 Mrs. Thitaporn P. [email protected] PREMIER FOOD AND MACHINERY.CO.LTD 68/76-77 Moo5 Rama2Rd., Jomthong Dist, Bangkok 10150 Thailand Tel (66-2)476-6901, 477-1045, 476-9690, 877-0525-7 Textured vegetable protein is a versatile substance; different forms allow it to take on the texture of whatever ground meat it is substituting. Using TVP, one can make vegetarian or vegan versions of traditionally meat-based dishes, such as chili con carne, spaghetti Bolognese, sloppy joes, tacos, burgers, or burritos. -

The Versatile Veggie Burger Home-Made Veggie (Or ‘Vegetarian’) Burgers Are a Delicious and Nutritious Alternative to the Traditional Hamburger
From the Food Bank Kitchen The Versatile Veggie Burger Home-made veggie (or ‘vegetarian’) burgers are a delicious and nutritious alternative to the traditional hamburger. Making veggie burgers instead of beef hamburgers is an easy way to eat less calories, fat, and cholesterol, while including more lean protein, extra fiber, vitamins and minerals into your diet. The American Cancer Society recommends cutting down on red meats (hamburgers, hot dogs, and deli meats) to reduce the risk of developing certain cancers. Eating lean, vegetarian sources of protein instead of red meat may also lower your risk of developing chronic diseases like obesity and heart disease. It is easy and inexpensive to make veggie burgers at home, and you can find frozen veggie burgers at just about any supermarket, even WalMart! See next page for the top-rated and healthiest store-bought veggie burger brands. The best thing about making your own veggie burgers is that they can be made with just about anything you might have in the kitchen, with low-cost ingredients that are full of fiber and protein. Examples include using canned or dried beans like black beans, pinto beans, red or white beans and lentils; grains like brown rice, oatmeal, or flour and bread crumbs; frozen and canned vegetables like carrots, potatoes, onions, and peppers, and chopped nuts and sunflower seeds. You can make it mild or spicy, according to your taste. If you don’t have all of the ingredients in the recipes below, try substituting the missing item with another one listed above! Veggie Burgers can be baked, grilled, or sautéed in a fry pan on the stove, and eaten on a bun, in a pita, or between slices of bread with lettuce, tomato, ketchup and mustard, just like regular hamburgers! Veggie burgers are also great topped with hummus or salsa, eaten with rice, or crumbled on a salad. -

Simply Soy Bingo Kit
Lesson Plan Jayme Ericson, Dietetic Intern Julie Garden-Robinson, Ph.D., R.D., L.R.D., Food and Nutrition Specialist Target group All ages Time needed 30 to 50 minutes, depending on activities Objectives Slide 1 - • Participants will be able to identify food Introduction sources of soy. Introduce yourself and welcome participants. • Participants will know what counts as a Welcome to this informational session about soy and its serving of soy. uses in our food supply. • Participants will know MyPlate recommendations for soy. Soy is pivotal to our society and used for much more than just food in our world. • Participants will know how to prepare soy. Let’s consider a few facts to get us started: Preparation and Supplies • How many crayons can one acre of soybeans • Copies of handout: “Questions and produce? 82,000 crayons Answers About Soy Foods,” FN1786 • What percent of all daily newspapers in the U.S. are • Copies of recipes printed using soybean oil? 50 percent • Simply Soy Bingo Kit • Food packages with soy-containing ingredients • Taste testing: Prepare a recipe from this lesson for taste testing, soy nuts or soy products, such as edamame (optional). Slide 2 - Products from soy Question: Which of these products are made from soy? All of the products are made from soy. A more detailed list is provided on your handout, and we will discuss it later. North Dakota State University Optional activity: Distribute food packages and look for Fargo, North Dakota the health claim and allergen statement. January 2016 Slide 3 - What is soy? Soy is a plant native to Asia and has been a staple in the Asian diet for more than 5,000 years. -

Soy Free Diet Avoiding Soy
SOY FREE DIET AVOIDING SOY An allergy to soy is common in babies and young children, studies show that often children outgrow a soy allergy by age 3 years and the majority by age 10. Soybeans are a member of the legume family; examples of other legumes include beans, peas, lentils and peanut. It is important to remember that children with a soy allergy are not necessarily allergic to other legumes, request more clarification from your allergist if you are concerned. Children with a soy allergy may have nausea, vomiting, abdominal pain, diarrhea, bloody stool, difficulty breathing, and or a skin reaction after eating or drinking soy products. These symptoms can be avoided by following a soy free diet. What foods are not allowed on a soy free diet? Soy beans and edamame Soy products, including tofu, miso, natto, soy sauce (including sho yu, tamari), soy milk/creamer/ice cream/yogurt, soy nuts and soy protein, tempeh, textured vegetable protein (TVP) Caution with processed foods - soy is widely used manufactured food products – remember to carefully read labels. o Soy products and derivatives can be found in many foods, including baked goods, canned tuna and meat, cereals, cookies, crackers, high-protein energy bars, drinks and snacks, infant formulas, low- fat peanut butter, processed meats, sauces, chips, canned broths and soups, condiments and salad dressings (Bragg’s Liquid Aminos) USE EXTRA CAUTION WITH ASIAN CUISINE: Asian cuisine are considered high-risk for people with soy allergy due to the common use of soy as an ingredient and the possibility of cross-contamination, even if a soy-free item is ordered. -

Soy Allergy Diet Avoid All Sources of Soy Protein
FAIRFAX 3903-A Fair Ridge Drive • Fairfax, VA 22033 • 703 648 0030 • fax: 703 648 9028 WOODBRIDGE 1952 Opitz Blvd • Woodbridge, VA 22191 • 703 494 7849 • fax: 703 494 8730 wheezefree.com SOY ALLERGY DIET AVOID ALL SOURCES OF SOY PROTEIN Avoiding products made with soy is difficult. Soybeans have become a major part of processed food products in our country. Soybeans and soybean products are found in baked goods, canned tuna, cereals, crackers, infant formulas, sauces, and soups. In the processing of most soybean oils, the protein portion is removed. Studies have shown that most people with a soy allergy my safely eat soy lecithin and soybean oil. READ FOOD LABELS Knowing how to read a food label will help you avoid problems caused by soybean protein in foods. Terms that mean the product does contain soybean protein: edamame soy bean (granules, curd) hydrolyzed soy protein soy protein miso Tamari shoyu sauce Tempeh soy (albumin, fiber, flour, grits, nuts, milk, sprouts) textured vegetable soy protein (concentrate, isolate) protein (TVP) soy sauce tofu soya Terms that may mean the product contains soybean protein: flavoring (natural and artificial) vegetable gum vegetable broth vegetable starch PROVIDE MISSING NUTRIENTS Soybeans alone aren’t a major food in the diet, but because they are in so many products, eliminating all those foods can result in a vitamin deficiency. As a precaution, give your child a daily vitamin pill. Work with a dietitian to be sure your child’s nutritional needs are met.. -

All About Sausage Made from Plants
ALL ABOUT SAUSAGE MADE FROM PLANTS There’s nothing quite like Impossible™ Sausage Made From Plants. And as hosts, servers, and restaurant managers, you might get some questions from guests like, “are you sure this is made from plants?”. Here’s a little cheat sheet that covers some of the most common questions: [If ofering an Impossible menu item to a vegan customer, be WHAT IS IMPOSSIBLE SAUSAGE sure to check with the kitchen staf on whether the dish can be MADE FROM PLANTS? prepared entirely plant-based. For example, anything containing cheese, butter, and eggs would have to be removed for vegans.] It’s delicious Sausage Made From Plants for meat lovers! You’ve gotta taste it to believe it! Many consumers actually prefer the favor over pork sausage. DOES IT CONTAIN ALLERGENS? WHY SHOULD I TRY IMPOSSIBLE It’s plant-based, nut-free, and dairy-free. It contains soy. SAUSAGE MADE FROM PLANTS? NUTRITIONALLY, HOW DOES IT • It’s unbelievably delicious (preferred over COMPARE TO PORK SAUSAGE? the leading brand of pork sausage!).* • It’s got all the protein of pork sausage, with 45% fewer It matches the protein levels in pork sausage and compared calories, 60% less total fat and 0 mg of cholesterol. with the leading sausage brand has: • It’s made from plants. • 45% fewer calories • It’s made for people who love meat • 60% less total fat • It’s way better for the planet than sausage from pigs • 0 mg cholesterol (in 1.6oz of Impossible™ Sausage) — because it uses a fraction of the land and water, and generates far fewer greenhouse gas emissions. -
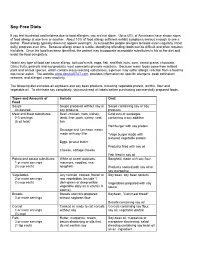
Soy-Free Diets
Soy-Free Diets If you feel frustrated and helpless due to food allergies, you are not alone. Up to 60% of Americans have shown signs of food allergy at one time or another. About 10% of food allergy sufferers exhibit symptoms serious enough to see a doctor. Food allergy typically does not appear overnight. In susceptible people allergies to foods eaten regularly (if not daily) progress over time. Because allergy onset is subtle, identifying offending foods can be difficult and often requires trial diets. Once the food has been identified, the patient may incorporate acceptable substitutes in his or her diet and avoid the food completely. Nearly any type of food can cause allergy, but cow’s milk, eggs, fish, shellfish, nuts, corn, cereal grains, chocolate, Citrus fruits, peanuts and soy products most commonly provoke reactions. Because many foods come from related plant and animal species, which contain cross-reacting substances, a person may suffer allergic reaction from a food ha has never eaten. The website www.dpcAlaSTAT.com provides information on specific allergens, peak pollination seasons, and allergen cross-reactivity. The following diet excludes all soybeans and soy bean products, including vegetable protein, lecithin, flour and vegetable oil. To eliminate soy completely, you must read all labels before purchasing commercially prepared foods. Types and Amounts of Include Omit Food Soups Soups prepared without soy or Soups containing soy or soy As desired soy products products Meat and Meat substitutes Beef, chicken, ham, kidney, -

CHAPTER 1 INTRODUCTION 1.1 Background of Study Tempeh Is A
CHAPTER 1 INTRODUCTION 1.1 Background of Study Tempeh is a traditional fermented soybean food. It is a fermented food made by the controlled fermentation of cooked soybeans with a Rhizopus mould as the tempeh starter. The tempeh fermentation by the Rhizopus mould binds the soybeans into compact white cake (Tempeh 2009,25 June). It is made by cooking and dehulling of soybeans and inoculation with different strains of Rhizopus (R. oligosporus, R. oryzae, and R. stolonifer) which will lead to solid substrate fermentation (Steinkraus K.H., 1983). Fermentation also influences the content of desirable constituents such as vitamins, protein and fatty acids (Baumann U., 1995). Tempeh provides the staple food for a large population in Indonesia and Malaysia. Like tofu, tempeh is made from soybeans, but tempeh is a whole soybean product with different nutritional characteristics and also different textural qualities. Tempeh’s fermentation process and its retention of the whole bean give it higher content of protein, dietary fiber and vitamins compared to tofu, as well as firmer texture and also with stronger flavor. Because of its nutritional value, tempeh is used worldwide in vegetarian cuisine. Tempeh is a low cost nutritious food and can be consumed by all socio-economic groups. 2 Figure 1.1 : A piece of uncooked tempe Table 1.1: Nutritional table for 100g of Tempe Water 54.9 g Energy 199 kcal Energy 833 kJ Protein 19.0 g Fat 7.7 g Sat. fatty acids 1.11 g Mono-unsat. fatty acids 1.7 g Poly-unsat. fatty acids 4.3 g Carbohydrates 17.0 g Fiber -

Soy Allergy Avoidance List Hidden Names for Soy Compiled by Debra A
Soy Allergy Avoidance List Hidden Names for Soy Compiled by Debra A. Indorato RD, LDN, member of KFA’s Medical Advisory Team Foods covered by the FDA labeling laws that contain soy must be labeled in plain English to declare that it “contains soy.” However, there are many foods and products that are not covered by FDA allergen labeling laws, so it is still important to know how to read a label for soy ingredients. Products exempt from plain English labeling rules: foods that are not regulated by the FDA (tinyurl.com/KFA-FALCPA), cosmetics and personal care products, prescription and over-the-counter medications or supplements, pet food, toys and crafts. contain soy The following ingredients found on a label indicate the presence of soy protein. All labels should be read carefully before consuming a product, even if it has been used safely in the past. Bean curd Soya may contain soy Edamame (soybeans in pods) Soya Flour Artificial flavoring Hydrolyzed soy protein Soybeans Asian foods (e.g. Japanese, Kinnoko flour Soybean granules Chinese, Thai, etc.) Kyodofu (freeze dried tofu) Soybean curd Hydrolyzed plant protein Miso Soybean flour Hydrolyzed vegetable protein (HVP) Natto Soy lecithin* Natural flavoring Okara (soy pulp) Soybean paste Vegetable broth Shoyu sauce Supro Vegetable gum Soy albumin Tamari Vegetable starch Soy bran Tempeh Soy concentrate Teriyaki sauce should be safe Soy oil (except cold pressed, expeller Soy fiber Textured soy flour (TSF) pressed or extruded soybean oil) Soy flour Textured soy protein (TSP) Soy formula Textured vegetable protein (TVP) Vegetable oil derived from soy Soy grits Tofu These soy derivatives should be safe Soy milk Yakidofu for most soy-allergic individuals. -

Physicochemical Properties of Soy- and Pea-Based Imitation Sausage Patties ______
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Missouri: MOspace PHYSICOCHEMICAL PROPERTIES OF SOY- AND PEA-BASED IMITATION SAUSAGE PATTIES ______________________________________________________ A Thesis presented to the Faculty of the Graduate School at the University of Missouri _______________________________________________________ In Partial Fulfillment of the Requirements for the Degree Master of Science _____________________________________________________ by CHIH-YING LIN Dr. Fu-hung Hsieh, Thesis Supervisor MAY 2014 The undersigned, appointed by the dean of the Graduate School, have examined the thesis entitled PHYSICOCHEMICAL PROPERTIES OF SOY- AND PEA-BASED IMITATION SAUSAGE PATTIES presented by Chih-ying Lin a candidate for the degree of Master Science, and hereby certify that, in their opinion, it is worthy of acceptance. Dr. Fu-hung Hsieh, Department of Biological Engineering & Food Science Dr. Andrew Clarke, Department of Food Science Dr. Gang Yao, Department of Biological Engineering ACKNOWLEDGEMENTS On the way to acquiring my master degree, many friends, professors, faculty and laboratory specialists gave me a thousand hands toward the completion of my academic research. First of all, I would like to thank Dr. Fu-hung Hsieh and Senior Research Specialist Harold Huff, who supported and offered me the most when conducting the experiment. I would like to thank Dr. Andrew Clarke and Dr. Gang Yao being my committee members and gave suggestion and help during my study. I would like to have a further thank to Dr. Mark Ellersieck for his statistical assistance. I am thankful and appreciate Carla Roberts and Starsha Ferguson help on editing and proofreading my thesis. -

HIGH PROTEIN SOY ISOLATES Add High-Quality, Complete Protein with Ease
HIGH PROTEIN SOY ISOLATES Add high-quality, complete protein with ease CHS soy isolates are designed to meet the needs of progressive food, beverage and dietary supplement manufacturers seeking a highly purified form of soy protein. We offer a full line of trusted CHS soy isolate products that are ideal for diverse applications ranging from dry, blended beverages and protein bars to supplements, ready-to-drink beverages, and meat products. CHS uses a unique method designed to remove carbohydrates and other factors, and then dries the protein to preserve its highly functional properties, resulting in a uniquely clean tasting flavor profile that provides versatility and ease when formulating your applications. Reliable, quality soy proteins Ask us about specialized Typical Product Composition* At CHS, we take pride in soy isolates such as: producing a vast range of • Highly dispersible isolated soy Protein (mfb) 90% minimum quality ingredients from proteins – for ready-to-drink soybeans using state-of-the-art and dry blended beverages Moisture 6% maximum technology and equipment. • Low viscosity isolated soy Standard Plate 10,000 maximum Our isolated soy proteins are proteins – for ready-to-drink Count (per g) manufactured from North beverages, nutritional bars, Salmonella Negative American non-GMO soybeans and extruded nuggets (per 375 g) at our South Sioux City, Neb., and crisps E. coli (per g) <3 cfu/g USA facility. • High viscosity, high gelling, • CHS soy isolates are good emulsifier for * all isolates except those with added components such as calcium have 90% formulated to deliver excellent emulsified meats minimum protein functional properties tailored Key applications to a wide range of applications. -

Changes of Soybean Protein During Tofu Processing
foods Review Changes of Soybean Protein during Tofu Processing Xiangfei Guan 1,2 , Xuequn Zhong 1, Yuhao Lu 1, Xin Du 1, Rui Jia 1, Hansheng Li 2 and Minlian Zhang 1,* 1 Department of Chemical Engineering, Institute of Biochemical Engineering, Tsinghua University, Beijing 100084, China; [email protected] (X.G.); [email protected] (X.Z.); [email protected] (Y.L.); [email protected] (X.D.); [email protected] (R.J.) 2 School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 102488, China; [email protected] * Correspondence: [email protected]; Tel./Fax: +86-10-6279-5473 Abstract: Tofu has a long history of use and is rich in high-quality plant protein; however, its produc- tion process is relatively complicated. The tofu production process includes soybean pretreatment, soaking, grinding, boiling, pulping, pressing, and packing. Every step in this process has an impact on the soy protein and, ultimately, affects the quality of the tofu. Furthermore, soy protein gel is the basis for the formation of soy curd. This review summarizes the series of changes in the composition and structure of soy protein that occur during the processing of tofu (specifically, during the pressing, preservation, and packaging steps) and the effects of soybean varieties, storage conditions, soybean milk pretreatment, and coagulant types on the structure of soybean protein and the quality of tofu. Finally, we highlight the advantages and limitations of current research and provide directions for future research in tofu production. This review is aimed at providing a reference for research into and improvement of the production of tofu.