Effects of Doenjang, a Traditional Korean Soybean Paste, with High
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ACTA KOR ANA VOL. 11, NO. 3, DECEMBER 2008: 235–269 BOOK REVIEWS Human Decency. by Kong Ji Yŏng. Translated by Bruce And
A C T A K O R A N A VOL. 11, NO. 3, DECEMBER 2008: 235–269 BOOK REVIEWS Human Decency . By Kong Ji Yŏng. Translated by Bruce and Ju-Chan Fulton. Published by Jimoondang as part of the Portable Library of Korean Literature, Short Fiction no. 24. Oftentimes, contemporary Korean fiction takes the reader, sometimes by force, down the dark memory lane of modern Korean history. Because so many works of contemporary Korean fiction are highly referential, the reader is often enticed to consider a text’s social and political background. While this is not a unique phenomenon to Korea—works of literature from all corners of the world often deal to greater or lesser extents with pressing political and social issues—it seems striking that Korean works of fiction so frequently draw the attention of their readers to the political and social realities being depicted, and do so in a manner that come at the expense of poetic expression. Kong Ji Yŏng’s short stories, “Human Decency” and “Dreams,” included in the collection Human Decency published by Jimoondang, are both highly referential texts. Since her works are so fraught with political and social references, this review will first examine the historical context in which Kong Ji Yŏng writes, and will then examine the stories in greater detail. In the collection of essays, Twentieth Century Korean Literature , it is noted that “an important strand of thought regarding literature in 1980s Korea was that it should interrogate social concerns and articulate communal values... the eighties were, in many ways, an era of causes. -

Textured Soy Protein (TSP)
PREMIER FOOD AND MACHINERY.CO.LTD 68/76-77 Moo5 Rama2Rd., Jomthong Dist, Bangkok 10150 Thailand Tel (66-2)476-6901, 477-1045, 476-9690, 877-0525-7 Textured Soy Protein (TSP) Textured or texturized vegetable protein (TVP), also known as Textured Soy Protein (TSP), soy meat is a defatted soy flour product, a by-product of extracting soybean oil. It is often used as a meat analogue or meat extender. It is quick to cook, with a protein content comparable to certain meats. TVP can be made from soy flour or concentrate, containing 50% and 70% soy protein, respectively; they have a mild beany flavor. Both require rehydration before use, sometimes with flavoring added in the same step. TVP is extruded, causing a change in the structure of the soy protein which results in a fibrous, spongy matrix, similar in texture to meat. In its dehydrated form, TVP has a shelf life of longer than a year, but will spoil within several days after being hydrated. In its flaked form, it can be used similarly to ground meat. Tel. 02-476-6901, 081-6235918 Mrs. Thitaporn P. [email protected] PREMIER FOOD AND MACHINERY.CO.LTD 68/76-77 Moo5 Rama2Rd., Jomthong Dist, Bangkok 10150 Thailand Tel (66-2)476-6901, 477-1045, 476-9690, 877-0525-7 Textured vegetable protein is a versatile substance; different forms allow it to take on the texture of whatever ground meat it is substituting. Using TVP, one can make vegetarian or vegan versions of traditionally meat-based dishes, such as chili con carne, spaghetti Bolognese, sloppy joes, tacos, burgers, or burritos. -

Cuisines of Asia
WORLD CULINARY ARTS: Korea Recipes from Savoring the Best of World Flavors: Korea Copyright © 2014 The Culinary Institute of America All Rights Reserved This manual is published and copyrighted by The Culinary Institute of America. Copying, duplicating, selling or otherwise distributing this product is hereby expressly forbidden except by prior written consent of The Culinary Institute of America. SPICY BEEF SOUP YUKKAEJANG Yield: 2 gallons Ingredients Amounts Beef bones 15 lb. Beef, flank, trim, reserve fat 2½ lb. Water 3 gal. Onions, peeled, quartered 2 lb. Ginger, 1/8” slices 2 oz. All-purpose flour ½ cup Scallions, sliced thinly 1 Tbsp. Garlic, minced ½ Tbsp. Korean red pepper paste ½ cup Soybean paste, Korean 1 cup Light soy sauce 1 tsp. Cabbage, green, ¼” wide 4 cups chiffonade, 1” lengths Bean sprouts, cut into 1” lengths 2 cups Sesame oil 1 Tbsp. Kosher salt as needed Ground black pepper as needed Eggs, beaten lightly 4 ea. Method 1. The day prior to cooking, blanch the beef bones. Bring blanched bones and beef to a boil, lower to simmer. Remove beef when it is tender, plunge in cold water for 15 minutes. Pull into 1-inch length strips, refrigerate covered Add onions and ginger, simmer for an additional hour, or until proper flavor is achieved. Strain, cool, and store for following day (save fat skimmed off broth). 4. On the day of service, skim fat off broth - reserve, reheat. 5. Render beef fat, browning slightly. Strain, transfer ¼ cup of fat to stockpot (discard remaining fat), add flour to create roux, and cook for 5 minutes on low heat. -

The Versatile Veggie Burger Home-Made Veggie (Or ‘Vegetarian’) Burgers Are a Delicious and Nutritious Alternative to the Traditional Hamburger
From the Food Bank Kitchen The Versatile Veggie Burger Home-made veggie (or ‘vegetarian’) burgers are a delicious and nutritious alternative to the traditional hamburger. Making veggie burgers instead of beef hamburgers is an easy way to eat less calories, fat, and cholesterol, while including more lean protein, extra fiber, vitamins and minerals into your diet. The American Cancer Society recommends cutting down on red meats (hamburgers, hot dogs, and deli meats) to reduce the risk of developing certain cancers. Eating lean, vegetarian sources of protein instead of red meat may also lower your risk of developing chronic diseases like obesity and heart disease. It is easy and inexpensive to make veggie burgers at home, and you can find frozen veggie burgers at just about any supermarket, even WalMart! See next page for the top-rated and healthiest store-bought veggie burger brands. The best thing about making your own veggie burgers is that they can be made with just about anything you might have in the kitchen, with low-cost ingredients that are full of fiber and protein. Examples include using canned or dried beans like black beans, pinto beans, red or white beans and lentils; grains like brown rice, oatmeal, or flour and bread crumbs; frozen and canned vegetables like carrots, potatoes, onions, and peppers, and chopped nuts and sunflower seeds. You can make it mild or spicy, according to your taste. If you don’t have all of the ingredients in the recipes below, try substituting the missing item with another one listed above! Veggie Burgers can be baked, grilled, or sautéed in a fry pan on the stove, and eaten on a bun, in a pita, or between slices of bread with lettuce, tomato, ketchup and mustard, just like regular hamburgers! Veggie burgers are also great topped with hummus or salsa, eaten with rice, or crumbled on a salad. -

Simply Soy Bingo Kit
Lesson Plan Jayme Ericson, Dietetic Intern Julie Garden-Robinson, Ph.D., R.D., L.R.D., Food and Nutrition Specialist Target group All ages Time needed 30 to 50 minutes, depending on activities Objectives Slide 1 - • Participants will be able to identify food Introduction sources of soy. Introduce yourself and welcome participants. • Participants will know what counts as a Welcome to this informational session about soy and its serving of soy. uses in our food supply. • Participants will know MyPlate recommendations for soy. Soy is pivotal to our society and used for much more than just food in our world. • Participants will know how to prepare soy. Let’s consider a few facts to get us started: Preparation and Supplies • How many crayons can one acre of soybeans • Copies of handout: “Questions and produce? 82,000 crayons Answers About Soy Foods,” FN1786 • What percent of all daily newspapers in the U.S. are • Copies of recipes printed using soybean oil? 50 percent • Simply Soy Bingo Kit • Food packages with soy-containing ingredients • Taste testing: Prepare a recipe from this lesson for taste testing, soy nuts or soy products, such as edamame (optional). Slide 2 - Products from soy Question: Which of these products are made from soy? All of the products are made from soy. A more detailed list is provided on your handout, and we will discuss it later. North Dakota State University Optional activity: Distribute food packages and look for Fargo, North Dakota the health claim and allergen statement. January 2016 Slide 3 - What is soy? Soy is a plant native to Asia and has been a staple in the Asian diet for more than 5,000 years. -

Great Food, Great Stories from Korea
GREAT FOOD, GREAT STORIE FOOD, GREAT GREAT A Tableau of a Diamond Wedding Anniversary GOVERNMENT PUBLICATIONS This is a picture of an older couple from the 18th century repeating their wedding ceremony in celebration of their 60th anniversary. REGISTRATION NUMBER This painting vividly depicts a tableau in which their children offer up 11-1541000-001295-01 a cup of drink, wishing them health and longevity. The authorship of the painting is unknown, and the painting is currently housed in the National Museum of Korea. Designed to help foreigners understand Korean cuisine more easily and with greater accuracy, our <Korean Menu Guide> contains information on 154 Korean dishes in 10 languages. S <Korean Restaurant Guide 2011-Tokyo> introduces 34 excellent F Korean restaurants in the Greater Tokyo Area. ROM KOREA GREAT FOOD, GREAT STORIES FROM KOREA The Korean Food Foundation is a specialized GREAT FOOD, GREAT STORIES private organization that searches for new This book tells the many stories of Korean food, the rich flavors that have evolved generation dishes and conducts research on Korean cuisine after generation, meal after meal, for over several millennia on the Korean peninsula. in order to introduce Korean food and culinary A single dish usually leads to the creation of another through the expansion of time and space, FROM KOREA culture to the world, and support related making it impossible to count the exact number of dishes in the Korean cuisine. So, for this content development and marketing. <Korean Restaurant Guide 2011-Western Europe> (5 volumes in total) book, we have only included a selection of a hundred or so of the most representative. -

Soy Free Diet Avoiding Soy
SOY FREE DIET AVOIDING SOY An allergy to soy is common in babies and young children, studies show that often children outgrow a soy allergy by age 3 years and the majority by age 10. Soybeans are a member of the legume family; examples of other legumes include beans, peas, lentils and peanut. It is important to remember that children with a soy allergy are not necessarily allergic to other legumes, request more clarification from your allergist if you are concerned. Children with a soy allergy may have nausea, vomiting, abdominal pain, diarrhea, bloody stool, difficulty breathing, and or a skin reaction after eating or drinking soy products. These symptoms can be avoided by following a soy free diet. What foods are not allowed on a soy free diet? Soy beans and edamame Soy products, including tofu, miso, natto, soy sauce (including sho yu, tamari), soy milk/creamer/ice cream/yogurt, soy nuts and soy protein, tempeh, textured vegetable protein (TVP) Caution with processed foods - soy is widely used manufactured food products – remember to carefully read labels. o Soy products and derivatives can be found in many foods, including baked goods, canned tuna and meat, cereals, cookies, crackers, high-protein energy bars, drinks and snacks, infant formulas, low- fat peanut butter, processed meats, sauces, chips, canned broths and soups, condiments and salad dressings (Bragg’s Liquid Aminos) USE EXTRA CAUTION WITH ASIAN CUISINE: Asian cuisine are considered high-risk for people with soy allergy due to the common use of soy as an ingredient and the possibility of cross-contamination, even if a soy-free item is ordered. -

Effect of Rice (Oryza Sativa L.) Added to Meju (Korean Soybean Koji
Food and Nutrition Sciences, 2012, 3, 1167-1175 1167 http://dx.doi.org/10.4236/fns.2012.38154 Published Online August 2012 (http://www.SciRP.org/journal/fns) Effect of Rice (Oryza sativa L.) Added to Meju (Korean Soybean Koji) Dough on Variation of Nutritional Ingredients and Bacterial Community Diversity in Doenjang (Korean Soybean Paste) Bo Young Jeon, Doo Hyun Park* Department of Biological Engineering, Seokyeong University, Seoul, South Korea. Email: *[email protected] Received May 21st, 2012; revised July 2nd, 2012; accepted July 9th, 2012 ABSTRACT In this study, effect of rice added to meju dough was evaluated based on the variation of nutritional ingredients and bacterial community diversity in finished doenjang. The ratio of rice added to meju dough (cooked and crushed soybean before fermentation) was 0, 0.2, and 0.4 based on dry weight. Free amino acids, minerals, polyphenol, and total pheno- lic compounds relatively decreased by addition of rice. However, 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) scavenging and ferric ion reduction activity was not influenced. Organic acids, which are fermentation metabolites, sig- nificantly increased in proportion to percentage balance of rice. Seven and four volatile compounds (VCs) were de- tected in doenjang prepared without and with rice, respectively, and contents of VCs were significantly lower in the rice-supplemented doenjang. Bacterial community diversity was significantly increased by addition of rice to meju dough. Rice alters chemical composition, fermentation products, and bacterial diversity, but does not downgrade the nature of doenjang. Keywords: Soybean Koji; Soybean Paste; Rice; Antioxidant Activity; Bacterial Community 1. Introduction on the basis of dry weight, and also contains physiologi- cally functional compounds such as polyphenol, total Doenjang is a Korean soy paste that has been prepared phenolic compounds, isoflavones, vitamin E, saponin, traditionally by long-term ripening of meju under high and anthocyanin [5-7]. -

Soy Allergy Diet Avoid All Sources of Soy Protein
FAIRFAX 3903-A Fair Ridge Drive • Fairfax, VA 22033 • 703 648 0030 • fax: 703 648 9028 WOODBRIDGE 1952 Opitz Blvd • Woodbridge, VA 22191 • 703 494 7849 • fax: 703 494 8730 wheezefree.com SOY ALLERGY DIET AVOID ALL SOURCES OF SOY PROTEIN Avoiding products made with soy is difficult. Soybeans have become a major part of processed food products in our country. Soybeans and soybean products are found in baked goods, canned tuna, cereals, crackers, infant formulas, sauces, and soups. In the processing of most soybean oils, the protein portion is removed. Studies have shown that most people with a soy allergy my safely eat soy lecithin and soybean oil. READ FOOD LABELS Knowing how to read a food label will help you avoid problems caused by soybean protein in foods. Terms that mean the product does contain soybean protein: edamame soy bean (granules, curd) hydrolyzed soy protein soy protein miso Tamari shoyu sauce Tempeh soy (albumin, fiber, flour, grits, nuts, milk, sprouts) textured vegetable soy protein (concentrate, isolate) protein (TVP) soy sauce tofu soya Terms that may mean the product contains soybean protein: flavoring (natural and artificial) vegetable gum vegetable broth vegetable starch PROVIDE MISSING NUTRIENTS Soybeans alone aren’t a major food in the diet, but because they are in so many products, eliminating all those foods can result in a vitamin deficiency. As a precaution, give your child a daily vitamin pill. Work with a dietitian to be sure your child’s nutritional needs are met.. -

Title Layout
KIM’S KOREAN BBQ Haemool 김스 코리언 바베큐 Pa Jeon Pan Seared Mandu 김스 코리언 바베큐 KIM’S APPETIZERSKOREAN BBQ 전식 A-1. Japchae 잡채 Small 7.99 Vermicelli noodles stir fried with Large 10.99 beef & vegetables 만두 A-2. Mandu 6.99 Japchae Ddeobokki Korean style beef & vegetable dumplings (Pan Seared or Fried) A-3. Haemool Pa Jeon 해물 파전 12.99 Korean seafood pancake filled with various seafood & green onions (CONTAINS SHELLFISH) A-4. Ojingeo Twigim 오징어 튀김 14.99 BINGSU 빙수 Korean style fried squid (calamari) Shaved ice dessert with sweet topping. (CONTAINS EGGS) A-5. Ddeobokki 떡볶기 7.99 xx Spicy rice cakes and boiled egg with fish cakes stir-fried in a spicy red pepper sauce Mango Bingsu Patbingsu A-6. Kimchi Jeon 김치 전 12.99 Kimchi pancake made with kimchi, onions, & green onions x Mild Spicy xx Medium Spicy xxx Extra Spicy Strawberry Bingsu Blue-Berry Bingsu ENTREES 정식 김스 코리언 바베큐 KIM’S KOREAN Dak Bulgogi GRILLBBQ 구이 1. Galbisal 갈비살 26.99 Marinated BBQ beef boneless short rib with onions This menu item is available as an optional entrée. You may grill it at the table (two or more orders required) Add Romaine lettuce 1.99 2. Sam Gyeob Sal Gui 삼겹살 구이 19.99 Thick sliced barbeque-style pork belly This menu item is available as an optional entrée. You may grill it at the table (two or more orders required) 3. Bulgogi 불고기 17.99 Marinated grilled shredded beef & onions x Mild김스 Spicy 코리언 xx Medium 바베큐 Spicy xxx KIM’S Extra Spicy (spicy available upon request) This menu item is available as an optional entrée. -

All About Sausage Made from Plants
ALL ABOUT SAUSAGE MADE FROM PLANTS There’s nothing quite like Impossible™ Sausage Made From Plants. And as hosts, servers, and restaurant managers, you might get some questions from guests like, “are you sure this is made from plants?”. Here’s a little cheat sheet that covers some of the most common questions: [If ofering an Impossible menu item to a vegan customer, be WHAT IS IMPOSSIBLE SAUSAGE sure to check with the kitchen staf on whether the dish can be MADE FROM PLANTS? prepared entirely plant-based. For example, anything containing cheese, butter, and eggs would have to be removed for vegans.] It’s delicious Sausage Made From Plants for meat lovers! You’ve gotta taste it to believe it! Many consumers actually prefer the favor over pork sausage. DOES IT CONTAIN ALLERGENS? WHY SHOULD I TRY IMPOSSIBLE It’s plant-based, nut-free, and dairy-free. It contains soy. SAUSAGE MADE FROM PLANTS? NUTRITIONALLY, HOW DOES IT • It’s unbelievably delicious (preferred over COMPARE TO PORK SAUSAGE? the leading brand of pork sausage!).* • It’s got all the protein of pork sausage, with 45% fewer It matches the protein levels in pork sausage and compared calories, 60% less total fat and 0 mg of cholesterol. with the leading sausage brand has: • It’s made from plants. • 45% fewer calories • It’s made for people who love meat • 60% less total fat • It’s way better for the planet than sausage from pigs • 0 mg cholesterol (in 1.6oz of Impossible™ Sausage) — because it uses a fraction of the land and water, and generates far fewer greenhouse gas emissions. -
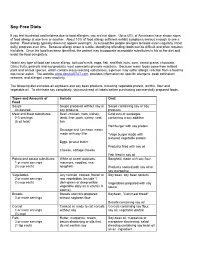
Soy-Free Diets
Soy-Free Diets If you feel frustrated and helpless due to food allergies, you are not alone. Up to 60% of Americans have shown signs of food allergy at one time or another. About 10% of food allergy sufferers exhibit symptoms serious enough to see a doctor. Food allergy typically does not appear overnight. In susceptible people allergies to foods eaten regularly (if not daily) progress over time. Because allergy onset is subtle, identifying offending foods can be difficult and often requires trial diets. Once the food has been identified, the patient may incorporate acceptable substitutes in his or her diet and avoid the food completely. Nearly any type of food can cause allergy, but cow’s milk, eggs, fish, shellfish, nuts, corn, cereal grains, chocolate, Citrus fruits, peanuts and soy products most commonly provoke reactions. Because many foods come from related plant and animal species, which contain cross-reacting substances, a person may suffer allergic reaction from a food ha has never eaten. The website www.dpcAlaSTAT.com provides information on specific allergens, peak pollination seasons, and allergen cross-reactivity. The following diet excludes all soybeans and soy bean products, including vegetable protein, lecithin, flour and vegetable oil. To eliminate soy completely, you must read all labels before purchasing commercially prepared foods. Types and Amounts of Include Omit Food Soups Soups prepared without soy or Soups containing soy or soy As desired soy products products Meat and Meat substitutes Beef, chicken, ham, kidney,