Asiaticoside Produces an Antidepressant‑Like Effect in a Chronic Unpredictable Mild Stress Model of Depression in Mice, Invol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Produzent Adresse Land Allplast Bangladesh Ltd
Zeitraum - Produzenten mit einem Liefertermin zwischen 01.01.2020 und 31.12.2020 Produzent Adresse Land Allplast Bangladesh Ltd. Mulgaon, Kaliganj, Gazipur, Rfl Industrial Park Rip, Mulgaon, Sandanpara, Kaligonj, Gazipur, Dhaka Bangladesh Bengal Plastics Ltd. (Unit - 3) Yearpur, Zirabo Bazar, Savar, Dhaka Bangladesh Durable Plastic Ltd. Mulgaon, Kaligonj, Gazipur, Dhaka Bangladesh HKD International (Cepz) Ltd. Plot # 49-52, Sector # 8, Cepz, Chittagong Bangladesh Lhotse (Bd) Ltd. Plot No. 60 & 61, Sector -3, Karnaphuli Export Processing Zone, North Potenga, Chittagong Bangladesh Plastoflex Doo Branilaca Grada Bb, Gračanica, Federacija Bosne I H Bosnia-Herz. ASF Sporting Goods Co., Ltd. Km 38.5, National Road No. 3, Thlork Village, Chonrok Commune, Konrrg Pisey, Kampong Spueu Cambodia Powerjet Home Product (Cambodia) Co., Ltd. Manhattan (Svay Rieng) Special Economic Zone, National Road 1, Sangkat Bavet, Krong Bavet, Svaay Rieng Cambodia AJS Electronics Ltd. 1st Floor, No. 3 Road 4, Dawei, Xinqiao, Xinqiao Community, Xinqiao Street, Baoan District, Shenzhen, Guangdong China AP Group (China) Co., Ltd. Ap Industry Garden, Quetang East District, Jinjiang, Fujian China Ability Technology (Dong Guan) Co., Ltd. Songbai Road East, Huanan Industrial Area, Liaobu Town, Donggguan, Guangdong China Anhui Goldmen Industry & Trading Co., Ltd. A-14, Zongyang Industrial Park, Tongling, Anhui China Aold Electronic Ltd. Near The Dahou Viaduct, Tianxin Industrial District, Dahou Village, Xiegang Town, Dongguan, Guangdong China Aurolite Electrical (Panyu Guangzhou) Ltd. Jinsheng Road No. 1, Jinhu Industrial Zone, Hualong, Panyu District, Guangzhou, Guangdong China Avita (Wujiang) Co., Ltd. No. 858, Jiaotong Road, Wujiang Economic Development Zone, Suzhou, Jiangsu China Bada Mechanical & Electrical Co., Ltd. No. 8 Yumeng Road, Ruian Economic Development Zone, Ruian, Zhejiang China Betec Group Ltd. -

Congressional-Executive Commission on China Annual
CONGRESSIONAL-EXECUTIVE COMMISSION ON CHINA ANNUAL REPORT 2016 ONE HUNDRED FOURTEENTH CONGRESS SECOND SESSION OCTOBER 6, 2016 Printed for the use of the Congressional-Executive Commission on China ( Available via the World Wide Web: http://www.cecc.gov U.S. GOVERNMENT PUBLISHING OFFICE 21–471 PDF WASHINGTON : 2016 For sale by the Superintendent of Documents, U.S. Government Publishing Office Internet: bookstore.gpo.gov Phone: toll free (866) 512–1800; DC area (202) 512–1800 Fax: (202) 512–2104 Mail: Stop IDCC, Washington, DC 20402–0001 VerDate Mar 15 2010 19:58 Oct 05, 2016 Jkt 000000 PO 00000 Frm 00003 Fmt 5011 Sfmt 5011 U:\DOCS\AR16 NEW\21471.TXT DEIDRE CONGRESSIONAL-EXECUTIVE COMMISSION ON CHINA LEGISLATIVE BRANCH COMMISSIONERS House Senate CHRISTOPHER H. SMITH, New Jersey, MARCO RUBIO, Florida, Cochairman Chairman JAMES LANKFORD, Oklahoma ROBERT PITTENGER, North Carolina TOM COTTON, Arkansas TRENT FRANKS, Arizona STEVE DAINES, Montana RANDY HULTGREN, Illinois BEN SASSE, Nebraska DIANE BLACK, Tennessee DIANNE FEINSTEIN, California TIMOTHY J. WALZ, Minnesota JEFF MERKLEY, Oregon MARCY KAPTUR, Ohio GARY PETERS, Michigan MICHAEL M. HONDA, California TED LIEU, California EXECUTIVE BRANCH COMMISSIONERS CHRISTOPHER P. LU, Department of Labor SARAH SEWALL, Department of State DANIEL R. RUSSEL, Department of State TOM MALINOWSKI, Department of State PAUL B. PROTIC, Staff Director ELYSE B. ANDERSON, Deputy Staff Director (II) VerDate Mar 15 2010 19:58 Oct 05, 2016 Jkt 000000 PO 00000 Frm 00004 Fmt 0486 Sfmt 0486 U:\DOCS\AR16 NEW\21471.TXT DEIDRE C O N T E N T S Page I. Executive Summary ............................................................................................. 1 Introduction ...................................................................................................... 1 Overview ............................................................................................................ 5 Recommendations to Congress and the Administration .............................. -

Table of Codes for Each Court of Each Level
Table of Codes for Each Court of Each Level Corresponding Type Chinese Court Region Court Name Administrative Name Code Code Area Supreme People’s Court 最高人民法院 最高法 Higher People's Court of 北京市高级人民 Beijing 京 110000 1 Beijing Municipality 法院 Municipality No. 1 Intermediate People's 北京市第一中级 京 01 2 Court of Beijing Municipality 人民法院 Shijingshan Shijingshan District People’s 北京市石景山区 京 0107 110107 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Haidian District of Haidian District People’s 北京市海淀区人 京 0108 110108 Beijing 1 Court of Beijing Municipality 民法院 Municipality Mentougou Mentougou District People’s 北京市门头沟区 京 0109 110109 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Changping Changping District People’s 北京市昌平区人 京 0114 110114 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Yanqing County People’s 延庆县人民法院 京 0229 110229 Yanqing County 1 Court No. 2 Intermediate People's 北京市第二中级 京 02 2 Court of Beijing Municipality 人民法院 Dongcheng Dongcheng District People’s 北京市东城区人 京 0101 110101 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Xicheng District Xicheng District People’s 北京市西城区人 京 0102 110102 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Fengtai District of Fengtai District People’s 北京市丰台区人 京 0106 110106 Beijing 1 Court of Beijing Municipality 民法院 Municipality 1 Fangshan District Fangshan District People’s 北京市房山区人 京 0111 110111 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Daxing District of Daxing District People’s 北京市大兴区人 京 0115 -

03032021 Nonfood Produzenten.Xlsx
Zeitraum - Produzenten mit einem Liefertermin zwischen 01.01.2020 und 31.12.2020 Produzent Adresse Land Allplast Bangladesh Ltd. Mulgaon, Kaliganj, Gazipur, Rfl Industrial Park Rip, Mulgaon, Sandanpara, Kaligonj, Gazipur, Dhaka Bangladesh Bengal Plastics Ltd. (Unit - 3) Yearpur, Zirabo Bazar, Savar, Dhaka Bangladesh Durable Plastic Ltd. Mulgaon, Kaligonj, Gazipur, Dhaka Bangladesh HKD International (Cepz) Ltd. Plot # 49-52, Sector # 8, Cepz, Chittagong Bangladesh Lhotse (Bd) Ltd. Plot No. 60 & 61, Sector -3, Karnaphuli Export Processing Zone, North Potenga, Chittagong Bangladesh Plastoflex Doo Branilaca Grada Bb, Gračanica, Federacija Bosne I H Bosnia-Herz. ASF Sporting Goods Co., Ltd. Km 38.5, National Road No. 3, Thlork Village, Chonrok Commune, Konrrg Pisey, Kampong Spueu Cambodia Powerjet Home Product (Cambodia) Co., Ltd. Manhattan (Svay Rieng) Special Economic Zone, National Road 1, Sangkat Bavet, Krong Bavet, Svaay Rieng Cambodia AJS Electronics Ltd. 1st Floor, No. 3 Road 4, Dawei, Xinqiao, Xinqiao Community, Xinqiao Street, Baoan District, Shenzhen, Guangdong China AP Group (China) Co., Ltd. Ap Industry Garden, Quetang East District, Jinjiang, Fujian China Ability Technology (Dong Guan) Co., Ltd. Songbai Road East, Huanan Industrial Area, Liaobu Town, Donggguan, Guangdong China Anhui Goldmen Industry & Trading Co., Ltd. A-14, Zongyang Industrial Park, Tongling, Anhui China Aold Electronic Ltd. Near The Dahou Viaduct, Tianxin Industrial District, Dahou Village, Xiegang Town, Dongguan, Guangdong China Aurolite Electrical (Panyu Guangzhou) Ltd. Jinsheng Road No. 1, Jinhu Industrial Zone, Hualong, Panyu District, Guangzhou, Guangdong China Avita (Wujiang) Co., Ltd. No. 858, Jiaotong Road, Wujiang Economic Development Zone, Suzhou, Jiangsu China Bada Mechanical & Electrical Co., Ltd. No. 8 Yumeng Road, Ruian Economic Development Zone, Ruian, Zhejiang China Betec Group Ltd. -
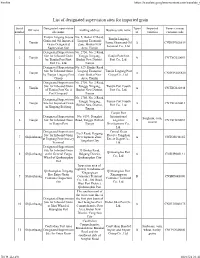
List of Designated Supervision Sites for Imported Grain
Firefox https://translate.googleusercontent.com/translate_f List of designated supervision sites for imported grain Serial Designated supervision Types Imported Venue (venue) Off zone mailing address Business unit name number site name of varieties customs code Tianjin Lingang Jiayue No. 5, Bohai 37 Road, Tianjin Lingang Grain and Oil Imported Lingang Economic 1 Tianjin Jiayue Grain and Oil A CNDGN02S619 Grain Designated Zone, Binhai New Terminal Co., Ltd. Supervision Site Area, Tianjin Designated Supervision No. 2750, No. 2 Road, Site for Inbound Grain Tanggu Xingang, Tianjin Port First 2 Tianjin A CNTXG020051 by Tianjin Port First Binhai New District, Port Co., Ltd. Port Co., Ltd. Tianjin Designated Supervision No. 529, Hunhe Road, Site for Inbound Grain Lingang Economic Tianjin Lingang Port 3 Tianjin A CNDGN02S620 by Tianjin Lingang Port Zone, Binhai New Group Co., Ltd. Group Area, Tianjin Designated Supervision No. 2750, No. 2 Road, Site for Inbound Grain Tanggu Xingang, Tianjin Port Fourth 4 Tianjin A CNTXG020448 of Tianjin Port No. 4 Binhai New District, Port Co., Ltd. Port Company Tianjin No. 2750, No. 2 Road, Designated Supervision Tanggu Xingang, Tianjin Port Fourth 5 Tianjin Site for Imported Grain A CNTXG020413 Binhai New District, Port Co., Ltd. in Xingang Beijiang Tianjin Tianjin Port Designated Supervision No. 6199, Donghai International Sorghum, corn, 6 Tianjin Site for Inbound Grain Road, Tanggu District, Logistics B CNTXG020051 sesame in Tianjin Port Tianjin Development Co., Ltd. Designated Supervision Central Grain No.9 Road, Haigang Site for Inbound Grain Reserve Tangshan 7 Shijiazhuang Development Zone, A CNTGS040165 at Jingtang Port Grocery Direct Depot Co., Tangshan City Terminal Ltd. -

Spatial and Temporal Evaluation of Ecological Footprint Intensity of Jiangsu Province at the County-Level Scale
International Journal of Environmental Research and Public Health Article Spatial and Temporal Evaluation of Ecological Footprint Intensity of Jiangsu Province at the County-Level Scale Decun Wu 1 and Jinping Liu 2,* 1 School of Public Administration and Sociology, Jiangsu Normal University, Xuzhou 221116, China; [email protected] 2 School of Economics and Management, China University of Mining and Technology, Xuzhou 221116, China * Correspondence: [email protected] Received: 10 September 2020; Accepted: 23 October 2020; Published: 26 October 2020 Abstract: Due to the high ecological pressure that exists in the process of rapid economic development in Jiangsu Province, it is necessary to evaluate its ecological footprint intensity (EFI). This article focuses on ecological footprint intensity analysis at the county scale. We used county-level data to evaluate the spatial distributions and temporal trends of the ecological footprint intensity in Jiangsu’s counties from 1995 to 2015. The temporal trends of counties are divided into five types: linear declining type, N-shape type, inverted-N type, U-shape type and inverted-U shape type. It was discovered that the proportions of the carbon footprint intensity were maintained or increased in most counties. Exploratory spatial data analysis shows that there was a certain regularity of the EFI spatial distributions, i.e., a gradient decrease from north to south, and there was a decline in the spatial heterogeneity of EFI in Jiangsu’s counties over time. The global Moran’s index (Moran’s I) and local spatial association index (LISA) are used to analyze both the global and local spatial correlation of EFIs among counties of Jiangsu Province. -

Global Factory List As of August 3Rd, 2020
Global Factory List as of August 3rd, 2020 Target is committed to providing increased supply chain transparency. To meet this objective, Target publishes a list of all tier one factories that produce our owned-brand products, national brand products where Target is the importer of record, as well as tier two apparel textile mills and wet processing facilities. Target partners with its vendors and suppliers to maintain an accurate factory list. The list below represents factories as of August 3rd, 2020. This list is subject to change and updates will be provided on a quarterly basis. Factory Name State/Province City Address AMERICAN SAMOA American Samoa Plant Pago Pago 368 Route 1,Tutuila Island ARGENTINA Angel Estrada Cla. S.A, Buenos Aires Ciudad de Buenos Aires Ruta Nacional N 38 Km. 1,155,Provincia de La Rioja AUSTRIA Tiroler Glashuette GmbH Werk: Schneegattern Oberosterreich Lengau Kobernauserwaldstrase 25, BAHRAIN WestPoint Home Bahrain W.L.L. Al Manamah (Al Asimah) Riffa Building #1912, Road # 5146, Block 951,South Alba Industrial Area, Askar BANGLADESH Campex (BD) Limited Chittagong zila Chattogram Building-FS SFB#06, Sector#01, Road#02, Chittagong Export Processing Zone,, Canvas Garments (Pvt.) Ltd Chittagong zila Chattogram 301, North Baizid Bostami Road,,Nasirabad I/A, Canvas Building Chittagong Asian Apparels Chittagong zila Chattogram 132 Nasirabad Indstrial Area,Chattogram Clifton Cotton Mills Ltd Chittagong zila Chattogram CDA plot no-D28,28-d/2 Char Ragmatia Kalurghat, Clifton Textile Chittagong zila Chattogram 180 Nasirabad Industrial Area,Baizid Bostami Road Fashion Watch Limited Chittagong zila Chattogram 1363/A 1364 Askarabad, D.T. Road,Doublemoring, Chattogram, Bangladesh Fortune Apparels Ltd Chittagong zila Chattogram 135/142 Nasirabad Industrial Area,Chattogram KDS Garment Industries Ltd. -

Discharge of Acid-Containing Wastewater Case Facts
This case is created by Fanyu Wang and Dr. Kathinka Fürst. All copy rights are reserved. For more information, please visit Kathinkafurst.org Discharge of Acid-Containing Wastewater Case Facts In June 2016, Mr. Ding Peng, the prosecutor of Hezi City, was reviewing a case that the local police office referred to the prosecutor office. The potential defendant in this case, Mr. Wang, operated a small quartz processing plant in Siqiu Village, Hezi City between April 2015 and May 2016. Mr. Wang employed hydrochloric acid and hydrofluoric acid in pickling the quartz stones and discharged the waste pickle liquor without a pollution discharge license. As a result, a total of over 100 tons of acid-containing wastewater was discharged to Lixi Canal and the connected Liangpu river, causing damages to the waterbodies. The investigation on this case began after an environmental protection NGO in Hezi City discovered and reported the wastewater discharge by Mr. Wang to the Environmental Protection Bureau (EPB) of Hezi City in April 2016. The multiple tests by EPB on the wastewater and the surface water of Lixi Canal and the connected Liangpu river showed that the pH and fluoride values considerably exceeded the permissible emission standards; the highest pH value exceeded the emission standard by 4.38 times and the highest fluoride value exceeded the emission standard by 45.8 times. The Environmental Protection Science Research Institute of Hezi City estimated the resulting damage to the waterbodies to be equivalent to CNY 200,999. The police office confirmed that the processing plant had stopped production in May 2016; Mr. -

Lianyungang Xingyi Fasteners Co.,Ltd Declaration Of
LIANYUNGANG XINGYI FASTENERS CO.,LTD HAIZHOU BAY INDUSTRIAL NESTING ZONE, HAITOU TOWN, GANYU DISTRICT, LIAN YUN GANG CITY, JIANGSU PROVINCE, CHINA TEL: 86‐574‐86508139 FAX: 86‐574‐86508208 DECLARATION OF PERFORMANCE Issued in accordance with the CONSTRUCTION PRODUCTS REGULATIONS This is to certify that, in accordance with EU Regulation No.305/2011 NO.DoP‐2017‐08‐001 1. Unique Identification of Product type: High‐strength Structural Bolting Assemblies 2.ORDER No.: IR108132 Product range: Bolt‐EN15048‐1:2007‐M (12‐36)*(25‐800) ‐ 10.9,8.8 Nut‐EN15048‐1:2007‐M (12‐36) ‐ 10,8. Washer‐EN15048‐1:2007‐D (12‐36)‐100HV(minimum) 3. High‐strength Structural Bolting Assemblies for building construction or civil engineering. 4. Name:Lianyungang Xingyi Fasteners Co., LTD Address: South Jinxing Road,Haizhou Bay Industrial Zone,Haitou Town,Ganyu District,Lianyungang City,Jiangsu Province 5. Harmonized standard: BS EN 15048‐1:2007 6. EC Certificate of Conformity No.: 0038/CPR/SHA/QAC6041035/B LRV Address: 71 Fenchurch Street London EC3M 4BS UK (United Kingdom). 7.System or systems of assessment and verification of constancy of performance of the construction product as set out in CPR, Annex V: System 2+ 8. Declared performance: Harmonized Essential characteristics Performance technical Characteristics Bolts Tolerances on dimensions, form EN ISO 4759-1 and position For d 二 24 mm and l 二 10 d or 150mm:A For d >24 mm Percent elongation after fracture Minimum tensile strength Stress at 0,2 % non-proportional Stress under proof load EN ISO 898-1 Strength under -

CHINA the Church of Almighty God: Prisoners Database (1663 Cases)
CHINA The Church of Almighty God: Prisoners Database (1663 cases) Prison term: 15 years HE Zhexun Date of birth: On 18th September 1963 Date and place of arrest: On 10th March 2009, in Xuchang City, Henan Province Charges: Disturbing social order and using a Xie Jiao organization to undermine law enforcement because of being an upper-level leader of The Church of Almighty God in mainland China, who was responsible for the overall work of the church Statement of the defendant: He disagreed with the decision and said what he believed in is not a Xie Jiao. Court decision: In February 2010, he was sentenced to 15 years in prison by the Zhongyuan District People’s Court of Zhengzhou City, Henan Province. Place of imprisonment: No. 1 Prison of Henan Province Other information: He was regarded by the Chinese authorities as a major criminal of the state and had long been on the wanted list. To arrest him, authorities offered 500,000 RMB as a reward to informers who gave tips leading to his arrest to police. He was arrested at the home of a Christian in Xuchang City, Henan Province. Based on the information from a Christian serving his sentence in the same prison, HE Zhexun was imprisoned in a separate area and not allowed to contact other prisoners. XIE Gao, ZOU Yuxiong, SONG Xinling and GAO Qinlin were arrested in succession alongside him and sentenced to prison terms ranging from 11 to 12 years. Source: https://goo.gl/aGkHBj Prison term: 14 years MENG Xiumei Age: Forty-one years old Date and place of arrest: On 14th August 2014, in Xinjiang Uyghur Autonomous Region Charges: Using a Xie Jiao organization to undermine law enforcement because of being a leader of The Church of Almighty God and organizing gatherings for Christians and the work of preaching the gospel in Ili prefecture Statement of the defendant: She claimed that her act did not constitute crimes. -
Chinese Tug of War Association Add: No.9, Tiyuguan Road, Beijing, China, 100763 Tel: 86-10-87182169、87182171 Fax: 86-10-67133577 Email:[email protected]
Chinese Tug of War Association Add: No.9, Tiyuguan Road, Beijing, China, 100763 Tel: 86-10-87182169、87182171 Fax: 86-10-67133577 Email:[email protected] To: TWIF Member Countries and Regions From : Chinese Tug of War Association Re: China(Ganyu) International Tug of War Tournament 2017 & Test Event of WIC 2018(Xuzhou) Invitation Dear Colleagues, First of all, to extend my sincere greetings to you. On behalf of CTWA, it gives us great pleasure in inviting you to attend the China International Tug of War Tournament 2017 at GanYu of JiangSu Province from October 13rd to 16th,2017. Senior W540 & Senior Mix600 4×4 shall be included in the Tournament.The Chinese Tug of War Association hereby invite your teams to participate in. We will provide all participants 4day/3night (from October 13rd to 16st,2017) free accommodation and local transportation. The top 6 teams will share a total of 6,000USD in prize money. We will hold a Test Event of WIC 2018 in Xuzhou after the Ganyu Tournament. The teams who want to participate in will be sent to Xuzhou by the LOC on 15th Oct(3hours by bus). The test event will be held at the same venue of the WIC 2018. We will provide all participants 3day/2night (from October 15th to 17th,2017) free accommodation and local transportation. Your participation will make the tournament a successful and more wonderful.We are looking forward to welcome you in China Yours sincerely Mr. HE YI Secretary General Chinese Tug of War Association 2017 China·Ganyu International Tug of War Tournament Regulations 1. -
Chinese Mainland
Address List of Special Warehousing Service Note: The address marked in red are newly added address. (Effective date:October 1, 2021) Province / Directly- controlled City District/county Town, Sub-district and House Number Municipality / Autonomous Region/SAR B4-25, Gate 1, ProLogis Logistics Park, No.1 Tiedi Road, Anhui Province Hefei Shushan District High-tech Zone No.18 Tianzhushan Road, Longshan Sub-district, Wuhu Anhui Province Wuhu Jiujiang District Economic and Technological Development Zone Anhui Province Chuzhou Langya District Longji Leye Photovoltaic Co., Ltd., No.19 Huai'an Road 3/F, No.8 Building, South Area, Lixiang Innovation Park, Anhui Province Chuzhou Nanqiao District Chuzhou, 018 Township Road Anhui Province Chuzhou Nanqiao District No.19 Huai'an Road Yuanrong New Material Holding Co., Ltd., 50 Meters Anhui Province Hefei Shushan District Westward of Bridge of Intersection of Changning Avenue and Ningxi Road Anhui Province Hefei Yaohai District No.88 Dayu Road Anhui Province Hefei Yaohai District No.2177 Dongfang Avenue Beijing BOE Vision-Electronic Technology Co., Ltd., No. Anhui Province Hefei Yaohai District 2177 Dongfang Avenue Anhui Province Hefei Yaohai District No.668 Longzihu Road Anhui Province Hefei Yaohai District No. 668 Longzihu Road Anhui Province Hefei Yaohai District No.2177 Tongling North Road Anhui Province Hefei Yaohai District No.3166 Tongling North Road Anhui Province Hefei Yaohai District No.8 Xiangwang Road Anhui Province Wuhu Jiujiang District No. 8 Anshan Road Anhui Province Wuhu Jiujiang District