HFO-1234Yf) Combustion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Method for Producing Hydrofluoroolefin
(19) TZZ¥__T (11) EP 3 187 478 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 05.07.2017 Bulletin 2017/27 C07C 17/23 (2006.01) C07C 21/18 (2006.01) C07B 61/00 (2006.01) (21) Application number: 15836384.6 (86) International application number: (22) Date of filing: 24.08.2015 PCT/JP2015/073749 (87) International publication number: WO 2016/031778 (03.03.2016 Gazette 2016/09) (84) Designated Contracting States: • TERAZONO, Shinji AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Tokyo 100-8405 (JP) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • KANDA, Masahiro PL PT RO RS SE SI SK SM TR Tokyo 100-8405 (JP) Designated Extension States: • KAWAGUCHI, Satoshi BA ME Tokyo 100-8405 (JP) Designated Validation States: • KASAGAWA, Mitsugu MA Tokyo 100-8405 (JP) (30) Priority: 25.08.2014 JP 2014170503 (74) Representative: Müller-Boré & Partner Patentanwälte PartG mbB (71) Applicant: Asahi Glass Company, Limited Friedenheimer Brücke 21 Tokyo 100-8405 (JP) 80639 München (DE) (72) Inventors: • WANG, Xu Tokyo 100-8405 (JP) (54) METHOD FOR PRODUCING HYDROFLUOROOLEFIN (57) A method for producing a hydrofluoroolefin, which comprises reacting a chlorofluoroolefin represented by the following formula (1) with hydrogen in the presence of a platinum group metal catalyst supported on a carbon carrier, to obtain a hydrofluoroolefin represented by the following formula (2), wherein the carbon carrier has acidic functional groups, and the total acidic functional group amount in the carbon carrier is at most 50 mmol/g: CZX=CClY (1) wherein X is F or Cl, Y is F, Cl or H, and Z is F or CF 3; CZX’=CHY’ (2) wherein X’ is F when X is F, or X’ is H when X is Cl, Y’ is F when Y is F, or Y’ is H when Y is Cl or H, and Z is the same as Z in the formula (1). -

Experimental Measurement and Modelling of Vapor-Liquid
Experimental measurement and modelling of vapor-liquid equilibrium for 3,3,3- Trifluoropropene (R1243zf) and trans-1,3,3,3-Tetrafluoropropene (R1234ze(E)) binary system Zhiqiang Yang, Alain Valtz, Christophe Coquelet, Jiangtao Wu, Jian Lu To cite this version: Zhiqiang Yang, Alain Valtz, Christophe Coquelet, Jiangtao Wu, Jian Lu. Experimental measurement and modelling of vapor-liquid equilibrium for 3,3,3- Trifluoropropene (R1243zf) and trans-1,3,3,3- Tetrafluoropropene (R1234ze(E)) binary system. International Journal of Refrigeration, Elsevier, 2020, 120, pp.137-149. 10.1016/j.ijrefrig.2020.08.016. hal-02963711 HAL Id: hal-02963711 https://hal.archives-ouvertes.fr/hal-02963711 Submitted on 11 Oct 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Experimental Measurement and Modelling of Vapor-Liquid Equilibrium for 3,3,3- Trifluoropropene (R1243zf) and trans-1,3,3,3-Tetrafluoropropene (R1234ze(E)) Binary System Zhiqiang Yanga,c, Alain Valtzb, Christophe Coqueletb*, Jiangtao Wua, Jian Luc a Key Laboratory of Thermo-Fluid Science and Engineering, Ministry of Education, Xi’an Jiaotong University, Xi’an, 710049, China b Mines Paristech, PSL University, CTP -Centre of Thermodynamics of Processes, 35 rue Saint-Honoré, 77300 Fontainebleau, France c State Key Laboratory of Fluorine & Nitrogen Chemicals, Xi’an Modern Chemistry Research Institute, Xi’an, 710065, China *Corresponding author. -

Study on Environmental and Health Effects of HFO Refrigerants (Publication Number: M-917|2017)
Study on environmental and health effects of HFO refrigerants (Publication number: M-917|2017) Report prepared for the Norwegian Environment Agency 22 December 2017 Study on environmental and health effects of HFO refrigerants Final Report RPA Report – Assured quality Project reference / title J972 / NEA HFO refrigerants Report status Final report David Fleet Dr James Hanlon Authors Dr Kate Osborne Max La Vedrine Paul Ashford (Anthesis-Caleb) Approved for issue by Meg Postle Date of issue 22 December 2017 Disclaimer The views and propositions expressed herein are, unless otherwise stated, those of Risk & Policy Analysts and do not necessarily represent any official view of the Norwegian Environment Agency or any other organisation mentioned in this report. List of abbreviations A2L Mildly flammable refrigerants category A5; non-A5 Article 5 Developing countries; non-Article 5 Developed countries BAU Business As Usual Cefic European Chemical Industry Council CF3C(O)H Trifluoroacetaldehyde - CF3COO Trifluoroacetate CFC Chlorofluorocarbon CLP Classification, Labelling and Packaging CMR Carcinogenic, mutagenic or toxic to reproduction CO2 Carbon dioxide COF2 Carbonyl fluoride CSR Chemical Safety Report EC10 10% effective concentration (the concentration that causes the measured effect in 10% of organisms) EC25 25% effective concentration (the concentration that causes the measured effect in 25% of organisms) EC50 Half maximum response dose (the concentration that causes the measured effect in 50% of organisms) ECHA European Chemicals Agency -

List of Refrigerants
Refrigerant Table: Explanation and Glossary of Terms A reference list of refrigerants which is intended to provide an indication of the basic characteristics, properties and applications for a variety of refrigerants along with some replacement options was prepared upon the recommendation of HRAI’s Task Team on the Future of Refrigerants. This list is intended to assist contractors as a convenient reference when selecting suitable replacement/substitute refrigerants for those refrigerants (CFC and any HCFC) which are being phased out. While refrigerant manufacturers may indicate some products are suitable for retrofit applications, the contractor will need to consider the application in order to determine the extent of system modifications that may be required to accommodate the replacement refrigerant. Currently, one must verify with the Original Equipment Manufacturer (OEM) to determine compatibility of alternative refrigerants with the equipment, or have a Professional Engineer sign off. Unless otherwise specified, few if any replacement refrigerants in the table are certified as a direct drop‐in replacement, in fact most are certified for new equipment only and are not suitable for retrofit applications. Contractors would still have to determine which products are suitable for their particular application, if equipment replacement or retrofit options exist, which system modifications may be required and choose accordingly. Depending on the age of the existing equipment and the extent of system modifications required to accommodate an alternate refrigerant, it may prove to be more cost effective in the long run to replace equipment outright. As the phase down of certain refrigerants causes them to become scarcer, decreasing availability and increasing cost can be expected to result in the change‐out of entire systems to more energy efficient equipment and environmentally friendly refrigerants. -

RAISE EER Boost
Presentation with Glossary Laboratory Comparatives • Completed expanded comparative benchmarking of RAISE EER Boost refrigerant at Applied Research Laboratories “ARL” in Florida • Completed expanded benchmarking to include R404 A at Electric Power Research Institute “EPRI” in conjunction with Southern California Edison 2 EER Boost-L R134A % Better EER Boost-M R407C % Better EERA: 10.6 10 6.00% EERA: 21.9 14.8 47.97% SEERA: 9.2 8.7 5.75% SEERA: 19.2 13 47.69% EERB: 11.4 10.9 4.59% EERB: 26.1 19.6 33.16% SEERB: 10 9.5 5.26% SEERB: 22.8 17.2 32.56% EER Boost-M R404A % Better EER Boost-M R22 % Better EERA: 8.8 7.2 22.22% EERA: 11.7 9 30.00% SEERA: 7.7 6.3 22.22% SEERA: 10.3 7.9 30.38% EERB: 11.5 9.2 25.00% EERB:* 12.9 18.3 -29.51% SEERB: 10.1 8.1 24.69% SEERB:* 11.3 16.1 -29.81% *incorrect settings during test 3 EER Boost-L R-134A % Better EERA: 10.6 10 6.00% SEERA: 9.2 8.7 5.75% EERB: 11.4 10.9 4.59% SEERB: 10 9.5 5.26% 4 EER Boost-M R-404A % Better EERA: 8.8 7.2 22.22% SEERA: 7.7 6.3 22.22% EERB: 11.5 9.2 25.00% SEERB: 10.1 8.1 24.69% 5 EER Boost-M R407C % Better EERA: 21.9 14.8 47.97% SEERA: 19.2 13 47.69% EERB: 26.1 19.6 33.16% SEERB: 22.8 17.2 32.56% 6 EER Boost-M R-22 % Better EERA: 11.7 9 30.00% SEERA: 10.3 7.9 30.38% EERB:* 12.9 18.3 -29.51% SEERB:* 11.3 16.1 -29.81% *incorrect settings during test 7 RAISE EER Boost™ Replacements 8 Intellectual Property Development Preparing 3 new patent application filings for: • composition • method of manufacture • method of use Working with industry experts to determine the chemical structure and radiative forcing model of the RAISE EER Boost refrigerant. -

Survey of Selected Fluorinated Green- House Gases
Survey of selected fluorinated green- house gases Part of the LOUS-review Environmental project No. 1655, 2015 Title: Authors & contributors: Survey of selected fluorinated greenhouse gases Erik Hansen 1 Per Henrik Pedersen 2 Frans Møller Christensen 1 Karen Louise Feilberg 1 Marlies Warming 1 Published by: 1 COWI A/S, Denmark 2Danish Technological Institute The Danish Environmental Protection Agency Strandgade 29 1401 Copenhagen K Denmark www.mst.dk/english Year: ISBN no. 2015 978-87-93283-82-4 Disclaimer: When the occasion arises, the Danish Environmental Protection Agency will publish reports and papers concerning re- search and development projects within the environmental sector, financed by study grants provided by the Danish Envi- ronmental Protection Agency. It should be noted that such publications do not necessarily reflect the position or opinion of the Danish Environmental Protection Agency. However, publication does indicate that, in the opinion of the Danish Environmental Protection Agency, the content represents an important contribution to the debate surrounding Danish environmental policy. While the information provided in this report is believed to be accurate, the Danish Environmental Protection Agency disclaims any responsibility for possible inaccuracies or omissions and consequences that may flow from them. Neither the Danish Environmental Protection Agency nor COWI or any individual involved in the preparation of this publication shall be liable for any injury, loss, damage or prejudice of any kind that may be caused by persons who have acted based on their understanding of the information contained in this publication. Sources must be acknowledged. 2 Survey of selected fluorinated greenhouse gases Contents Preface ...................................................................................................................... 5 Summary and conclusions ........................................................................................ -
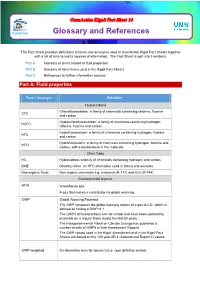
Glossary and References
OzonAction Kigali Fact Sheet 14 OzonAction Glossary and References This Fact Sheet provides definitions of terms and acronyms used in OzonAction Kigali Fact Sheets together with a list of links to useful sources of information. The Fact Sheet is split into 3 sections: Part A: Glossary of terms related to fluid properties Part B: Glossary of other terms used in the Kigali Fact Sheets Part C: References to further information sources Part A: Fluid properties Term / Acronym Definition Fluorocarbons Chlorofluorocarbon: a family of chemicals containing chlorine, fluorine CFC and carbon. Hydrochlorofluorocarbon: a family of chemicals containing hydrogen, HCFC chlorine, fluorine and carbon. Hydrofluorocarbon: a family of chemicals containing hydrogen, fluorine HFC and carbon. Hydrofluoroolefin: a family of chemicals containing hydrogen, fluorine and HFO carbon, with a double bond in the molecule. Other fluids HC Hydrocarbon: a family of chemicals containing hydrogen and carbon. DME Dimethyl ether: an HFC alternative used in foams and aerosols Non-organic fluids Non-organic chemicals e.g. ammonia (R-717) and CO2 (R-744) Environmental impacts GHG Greenhouse gas A gas that makes a contribution to global warming. GWP Global Warming Potential. The GWP compares the global warming impact of a gas to CO2 which is defined as having a GWP of 1. The GWPs of fluorocarbons are not certain and have been updated by scientists on a regular basis during the last 20 years. The Intergovernmental Panel on Climate Change has published a number of sets of GWPs in their Assessment Reports. The GWP values used in the Kigali Amendment and in the Kigali Fact Sheets are based on the 100 year AR 4 (Assessment Report 4) values. -

New Refrigerants for Vapour Compression Refrigeration and Heat Pump Systems
New refrigerants for vapour compression refrigeration and heat pump systems Evaluation in a context of the requirements set by the F-gas Regulation and the Paris Agreement goals. PAVEL MAKHNATCH Doctoral Thesis, 2019 KTH Royal Institute of Technology School of Industrial Engineering and Management Department of Energy Technology SE-100 44 Stockholm, Sweden TRITA-ITM-AVL 2019:25 ISBN: 978-91-7873-296-8 © Pavel Makhnatch, Stockholm 2019 Tryck: US-AB, Stockholm Akademisk avhandling som med tillstånd av KTH i Stockholm framlägges till offentlig granskning för avläggande av teknisk doktorsexamen tisdagen den 24 September 2019 klockan 13:00 i sal F3, KTH, Lindstedtsvägen 26, Stockholm, Sweden. To my mentors Abstract Given the global commitment to reduce the impact of fluorinated gases on climate and the regulations controlling their use in the European Union (EU), this thesis investigates new refrigerants that have been recently proposed to meet the demands of the refrigeration industry in the EU, which must satisfy the legislative requirements of the F-gas Regulation and facilitate meeting the goals of the Paris Agreement. The legislative requirements of the EU have intensified refrigerant development with reduced GWP. New refrigerants have been identified in the form of six new substances and 40 new refrigerant mixtures that have been added to the ANSI/ASHRAE 34 standard during a period following the proposal for the F-gas Regulation. New refrigerants have been theoretically analysed in comparison with the commonly used fluorinated refrigerants R134a, R404A and R410A. While new refrigerants provide a variation in operating parameters when used in a refrigeration, air conditioning or heat pump (RACHP) system, none of the new refrigerants can be considered being fully design compatible with R134a, R404A or R410A. -

Biotransformation of Trans-1,1,1,3-Tetrafluoropropene, 2,3,3,3-Tetrafluoropropene and 1,2,3,3,3-Pentafluoropropene"
Biotransformation of trans-1,1,1,3-tetrafluoropropene, 2,3,3,3-tetrafluoropropene and 1,2,3,3,3-pentafluoropropene Dissertation zur Erlangung des naturwissenschaftlichen Doktorgrades der Bayerischen Julius-Maximilians-Universität Würzburg vorgelegt von Paul Xaver Schuster aus Greifenberg Würzburg 2009 Eingereicht am: 04.11.2009 bei der Fakultät für Biologie der Bayerische Julius-Maximilians-Universität Würzburg 1. Gutachter: Prof. Dr. Wolfgang Dekant 2. Gutachter: Prof. Dr. Roland Benz der Dissertation 1. Prüfer: Prof. Dr. Wolfgang Dekant 2. Prüfer: Prof. Dr. Roland Benz 3. Prüfer:......................................................................................................................... des öffentlichen Promotionskolloquiums Datum des öffentlichen Promotionskolloquiums:............................................................ Doktorurkunde ausgehändigt am:.................................................................................. Contents I Contents page 1 Introduction 1 1.1 Environmental characteristics and metabolic susceptibility of chlorofluorocarbons and their replacements 1 1.2 Catabolism of glutathione S-conjugates 3 1.3 Bioactivation of haloalkenes 5 1.3.1 Cytochrome P450-dependent bioactivation 5 1.3.2 Glutathione-dependent bioactivation 7 1.4 Toxicity testing results with 2,3,3,3-tetrafluoropropene (HFO-1234yf) and trans-1,1,1,3-tetrafluoropropene (HFO-1234ze) 9 1.4.1 Toxicity testing of HFO-1234yf in rats and mice 10 1.4.2 Developmental toxicity testing of HFO-1234yf with rabbits 10 1.4.3 Toxicity testing -

Atmospheric Chemistry of Polyfluorinated Compounds: Long-Lived Greenhouse Gases and Sources of Perfluorinated Acids
ATMOSPHERIC CHEMISTRY OF POLYFLUORINATED COMPOUNDS: LONG-LIVED GREENHOUSE GASES AND SOURCES OF PERFLUORINATED ACIDS by Cora J. Young A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Department of Chemistry University of Toronto © Copyright by Cora J. Young 2010 Atmospheric chemistry of polyfluorinated compounds: Long-lived greenhouse gases and sources of perfluorinated acids Doctor of Philosophy Degree, 2010 Cora J. Young Department of Chemistry, University of Toronto ABSTRACT Fluorinated compounds are environmentally persistent and have been demonstrated to bioaccumulate and contribute to climate change. The focus of this work was to better understand the atmospheric chemistry of poly- and per-fluorinated compounds in order to appreciate their impacts on the environment. Several fluorinated compounds exist for which data on climate impacts do not exist. Radiative efficiencies (REs) and atmospheric lifetimes of two new long-lived greenhouse gases (LLGHGs) were determined using smog chamber techniques: perfluoropolyethers and perfluoroalkyl amines. Through this, it was observed that RE was not directly related to the number of carbon-fluorine bonds. A structure-activity relationship was created to allow the determination of RE solely from the chemical structure of the compound. Also, a novel method was developed to detect polyfluorinated LLGHGs in the atmosphere. Using carbotrap, thermal desorption and cryogenic extraction coupled to GC-MS, atmospheric measurements can be made for a number of previously undetected compounds. A perfluoroalkyl amine was detected in the atmosphere using this technique, which is the compound with the highest RE ever detected in the atmosphere. Perfluorocarboxylic acids (PFCAs) are water soluble and non-volatile, suggesting they are not susceptible to long-range transport. -

Environmental Management of Refrigeration Equipment
Supplement 16 Environmental management of refrigeration equipment Technical supplement to WHO Technical Report Series, No. 961, 2011 Annex 9: Model guidance for the storage and transport of time- and temperature-sensitive pharmaceutical products May 2015 © World Health Organization 2015 WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. -
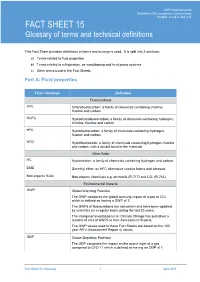
FACT SHEET 15 Glossary of Terms and Technical Definitions
UNEP Ozone Secretariat UNEP Ozone Secretariat – BackgroundWorkshop Material on HFC formanagement: HFC Workshop, technical 2015 issues Bangkok, 20 and 21 April 2015 FACT SHEET 15 Glossary of terms and technical definitions This Fact Sheet provides definitions of terms and acronyms used. It is split into 3 sections: a) Terms related to fluid properties b) Terms related to refrigeration, air-conditioning and heat pump systems c) Other terms used in the Fact Sheets Part A: Fluid properties Term / Acronym Definition Fluorocarbons CFC Chlorofluorocarbon: a family of chemicals containing chlorine, fluorine and carbon. HCFC Hydrochlorofluorocarbon: a family of chemicals containing hydrogen, chlorine, fluorine and carbon. HFC Hydrofluorocarbon: a family of chemicals containing hydrogen, fluorine and carbon. HFO Hydrofluoroolefin: a family of chemicals containing hydrogen, fluorine and carbon, with a double bond in the molecule. Other fluids HC Hydrocarbon: a family of chemicals containing hydrogen and carbon. DME Dimethyl ether: an HFC alternative used in foams and aerosols Non-organic fluids Non-organic chemicals e.g. ammonia (R-717) and CO2 (R-744) Environmental impacts GWP Global Warming Potential. The GWP compares the global warming impact of a gas to CO2 which is defined as having a GWP of 1. The GWPs of fluorocarbons are not certain and have been updated by scientists on a regular basis during the last 20 years. The Intergovernmental panel on Climate Change has published a number of sets of GWPs in their Assessment Reports. The GWP values used in these Fact Sheets are based on the 100 year AR 4 (Assessment Report 4) values. ODP Ozone Depletion Potential The ODP compares the impact on the ozone layer of a gas compared to CFC-11 which is defined as having an ODP of 1.